【】How to Write the Formula of Propane
Answer:
CH₃CH₂CH₃ is the chemical formula of propane.
Explanation:
Hello everyone!today we are going to learn about the formula of Propane.
Step-1:We know that prop means three.So propane has 3 carbon.
Step-2:Since propane is a organic compound.So it has C-H bond.To determine the number of hydrogen,we will use alkane general formula.Actually,propane is a member of alkane.The alkane general formula is CnH2n+2
Now put n=3,
You will get C3H8
So the formula of propane is
C3H8
This is molecular or chemical formula of propane.
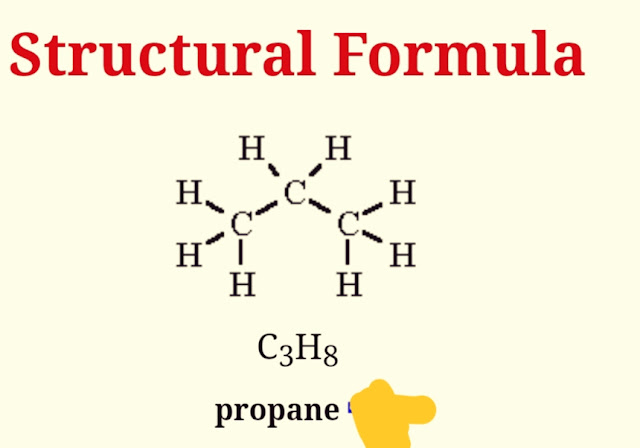
The structural formula of propane is given below:
 |
| structural formula of propane |
The condensed Structural formula of propane is given below:
CH₃CH₂CH₃
Here is the bond line structure of propane or skeletal formula of propane.
 |
| Bond Line Structure of Propane |
Continue








Comments
Post a Comment