[ ]What Is the Magnesium (Mg) Electron Configuration?
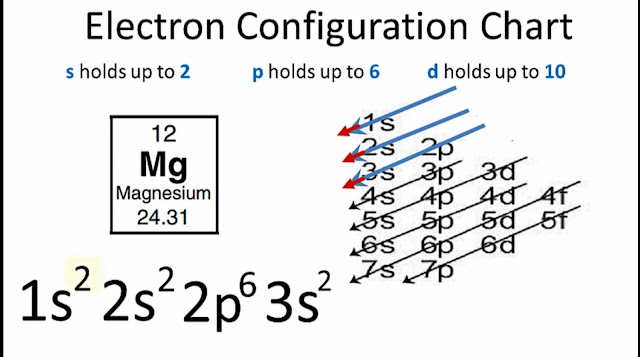
●Ground State Electron Configuration for Magnesium (Mg):1s² 2s² 2p⁶ 3s²
●Electron Configuration of a Neutral Atom of Magnesium:
1s² 2s² 2p⁶ 3s²
●Longhand Electron Configuration for Magnesium (Mg):1s² 2s² 2p⁶ 3s²
●Shorthand Electron Configuration for Magnesium (Mg):[Ne] 3s²
●Noble Gas Electron Configuration for Magnesium:[Ne] 3s²
●Unabbreviated Electron Configuration for Magnesium,
(Mg):1s² 2s² 2p⁶ 3s²
●Abbreviated Electron Configuration for Magnesium,
(Mg):[Ne] 3s²
Explanation:
Here is the way you can follow to write the electron configuration of magnesium (Mg) in just 5 steps.
Step-1:
To do electron configuration of magnesium
element, we have to know the atomic number of the magnesium
.The atomic number of magnesium element is 12.So magnesium has 12 electrons and 12 protons.
Look how I find the electron configuration for magnesium.
This process is applicable for all the elements of the periodic table.
Step-2:
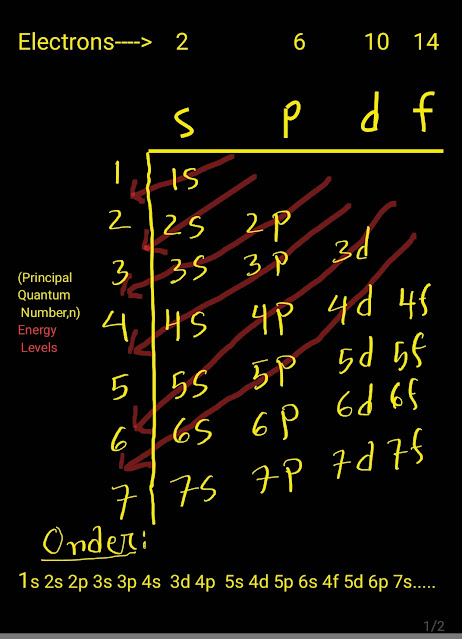
(How do you remember the order of electron configurations?)
Now we have to memorize the following sequence of the orbitals.
ss
ps ps
dps dps
fdps fdps fdps fdps....
In a line-
ss ps ps dps dps fdps fdps
fdps fdps fdps fdps.......
Step-3:
Now we have to first numer the s-orbital (start with 1s)like this:
1s2s
p3s p 4s
dp 5s dp 6s
fdp7s fdp 8s...... According to your need.
In a line-
1s2s p3s p 4s dp 5s dp 6s
To do electron configuration of magnesium
element, we have to know the atomic number of the magnesium
.The atomic number of magnesium element is 12.So magnesium has 12 electrons and 12 protons.
Look how I find the electron configuration for magnesium.
This process is applicable for all the elements of the periodic table.
Step-2:
(How do you remember the order of electron configurations?)
Now we have to memorize the following sequence of the orbitals.
ss
ps ps
dps dps
fdps fdps fdps fdps....
In a line-
ss ps ps dps dps fdps fdps
fdps fdps fdps fdps.......
Step-3:
Now we have to first numer the s-orbital (start with 1s)like this:
1s2s
p3s p 4s
dp 5s dp 6s
fdp7s fdp 8s...... According to your need.
In a line-
1s2s p3s p 4s dp 5s dp 6s
fdp7s fdp 8s........
Remember
● s-orbital start with 1s
● p-orbital start with 2p
● d-orbital start with 3d
● f-orbital start with 4f
Again,Now(second) we need number the p-orbital(start with 2p) like this:
ss
2ps 3ps
d 4ps d 5ps
fd6ps fd7ps fd8ps fd9ps....
In a line--
ss 2ps 3ps d4ps d5ps
fd6ps fd7ps fd8ps fd9ps....
Thirdly,we need number the d-orbital(start with 3d)like this:
ss
ps ps
3dps 4dps
f5dps f6dps f7dps f8dps....
In a line--
ss ps ps 3dps 4dps
f5dps f6dps f7dps f8dps....
Fourthly,number the f-orbital(start with 4f) like this:
ss
ps ps
dps dps
4fdps 5fdps 6fdps 7fdps....
In a line--
ss ps ps dps dps 4fdps 5fdps 6fdps 7fdps......
Fifltly,number s,p,d,f together like this....
1s2s
2p3s 3p4s
3d4p5s 4d5p6s
4f5d6p7s 5f6d7p8s
6f7d8p9s.........
In a line----
1s 2s 2p 3s 3p 4s 3d 4p 5s d 5p 6s 4f 5d 6p 7s 5f 6d 7p 8s
6f 7d 8p 9s.........
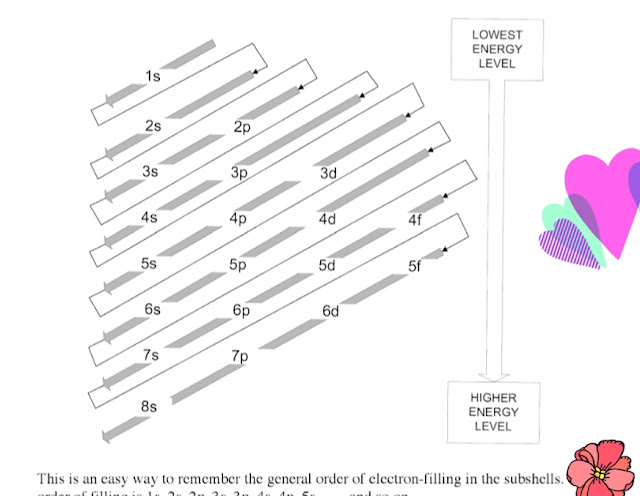 |
How do you remember the order of electron configurations?
|
Step-4:
(What is spdf in electron configuration?
think--)
Now we have to know that
•S-orbital can take maximum 2 electrons.
•p-orbital can take maximum 6 electrons.
•d-orbital can take maximum 10 electrons.
•f-orbital can take maximum 14 electrons.
 |
| How to Do or Find Electron Configuration |
Now Watch this Video
Then....
Step-5:
Now we know that magnesium has 12 electrons.
Electron configuration of magnesium will be...
s² s²
p⁶s² ps
dps dps
fdps fdps fdps....
So,we are getting..
s² s²
p⁶s²
Now number s-orbital according to above process.
1s² 2s²
p⁶3s²
Now number p-orbital according to above process.
1s² 2s²
2p⁶3s²

















Comments
Post a Comment