【】What Is the Electron Configuration with Step by Step Guides to write Electron Configurations of Atoms and Ions of Elements ?
Hello everyone! today I am going to discuss electron configuration. I hope you will enjoy this blog post.
The atomic number of an element indicates the number of protons in the nucleus of an atom of that element.Equal number of electrons are present outside the nucleus.
According to quantum mechanics, these electrons are arranged in different orbitals according to definite rules.
The arrangement of electrons in different orbitals of an atom is known as electron configuration.
Now watch this video about Electron configuration.
Here is the step by step guides to write electron configuration of neutral atoms and ions of different elements:
Electron configuration is guided by three rules,namely:
1) Pauli's exclusion principle
2)Aufbau principle
3)Hund's rule.
Here is the explanations:
In 1925 W. Pauli's formulated a principale about the arrangement of electrons in an atom. This is known as pauli's exclusion principle.
"One single atomic orbital(whose n ,l and m values are same) can accommodate maximum two electrons, whose spin must be opposite."
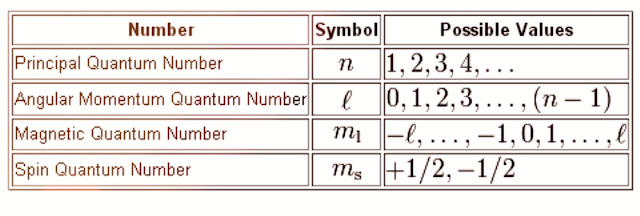
To understand the pauli's exclusion principle .You need to have the knowledge about the values of four quantum (n,l,m,s) numbers.
1)Pauli's Exclusion Principle
In 1925 W. Pauli's formulated a principale about the arrangement of electrons in an atom. This is known as pauli's exclusion principle.
The pauli's exclusion principle is given below:
To understand the pauli's exclusion principle .You need to have the knowledge about the values of four quantum (n,l,m,s) numbers.
For example,there are 2 electrons(1s²) in helium atom.The two electrons have the following values for four quantum numbers:
For 1st electron,e₁
n=1, l=0, m=0, s=+1/2
For 2nd electron,e₂
n=1, l=0, m=0, s=-1/2
Among the values of four quantum numbers of these two electrons, three values are identical but the fourth value is different.Hence pauli's exclusion principle is supported by helium atom.
So pauli's exclusion principle may also be stated as follows:
"One single atomic orbital(whose n ,l and m values are same) can accommodate maximum two electrons, whose spin must be opposite."
Application of Pauli's Exclusion Principle:
By using pauli's exclusion principle the possible maximum number of electrons in different orbitals can be calculated as follows:
•Whatever may be the value of n=1,2,3,4,....etc. when l=0.i.e.;in s-orbital, maximum electrons is 2.
•when l=1, i.e.,in three p-orbitals, maximum electrons is 6;
•when l=2, i.e.,in five d-orbitals maximum electrons is 10
•when l=3, i.e., in f-orbital maximum 14 electrons can be accommodated.
•In fact ,there are three p-orbitals (whose m are different),five d-orbitals and seven f-orbitals.
So pauli's exclusion principle may also be stated as follows:
"One single atomic orbital (whose n,l and m-values are same) can accommodate maximum two electrons,whose spin must be opposite."
The possible values of four quantum numbers and their shells, subshells or orbitals and maximum number of orbitals with their electrons are shown in the following table.

2)Aufbau principle
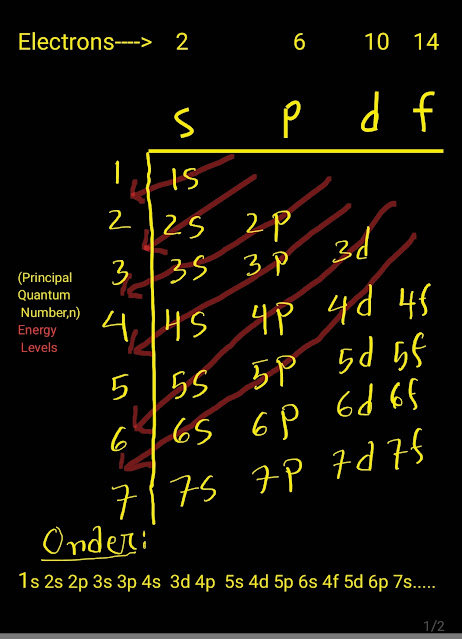
In an atom the electrons occupy different orbitals according to their increasing energy levels.
That means that electrons fast occupy the orbital of lowest energy and systematically then the orbitals of a higher energy level. This rules is called Aufbau principle.
The orbital, which has lower value of (n+l) has the lower energy and higher value of (n+l) has higher energy.Here 'n' and 'l' are principal and subsidiary quantum numbers.
The orbitals of different energy from lower to higher gradually are as follows:
1s,2s,2p,3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s....
How Do You Remember the order of Electron Configuration?
This gradational order can be remembered with the help of general scheme shown in figure and video.
School(s) School(s)
ss
public(p) School(s) public (p)school(s)
ps ps
daily(d) public(p) school (s)daily (d) public(p) school(s)
dps dps
fully(f) faily(d) public(p) school (s)Fully(f) faily(d) public(p) school (s).......
fdps fdps.......
So we are getting...
ss
ps ps
dps dps
fdps fdps.......
Now place number like this...(s start with 2s,p start with 2p ,d start 3d and f start with 4f
1ss
2ps ps
3dps dps
4fdps fdps.......
Then continue for s.....
1s2s
2p3s p4s
3dp5s dp6s
4fdp7s fdp8s.......
1s2s
2p3s 3p4s
3d4p5s d5p6s
4fd6p7s fd7p8s.......
Now continue for d-orbital...
1s2s
2p3s 3p4s
3d4p5s 4d5p6s
4f5d6p7s f6d7p8s.......
Now continue for f-orbital....
1s2s
2p3s 3p4s
3d4p5s 4d5p6s
4f5d6p7s 5f6d7p8s.......
1s2s2p3s 3p4s 3d4p5s 4d5p6s
4f5d6p7s 5f6d7p8s......
Determination of order of orbitals
To determine the comparative energy of different orbitals, the following rules are generally used:
Rule-1:Among several orbitals,the orbital whose (n + l) value is less, will be of lower energy.For example, among 3d,4s,4p orbitals:
*for 3d orbital:(n+l)=(3+2)=5
*for 4s orbital:(n+l)=(4+0)=4
*for 5p orbital:(n+l)=(5+1)=6
So among these three orbitals 4s orbitals will be of lowest energy,the 3d will be of higher energy and 5p will be of highest energy orbital.
Rule-2:If two or more orbitals possess same value of (n+l),then the orbital whose value of 'n' is less,will be of lower energy.For example among 3d,4p and 5s orbitals:
*for 3d orbital:(n+l)=(3+2)=5
*for 4p orbital:(n+l)=(4+1)=5
*for 5s orbital:(n+l)=(5+0)=5
For all these orbitals (n+l) value is equal to 5.Since for 3d orbital the value of n is less than the others,it will be of lowest energy;then comes 4p orbital and then comes 5s orbital having highest energy.
 |
| Electron Configuration |
Rules of Writing Electron Configuration
The electron configuration of any element is written in the ascending order of n.For example, we can write the electronic configuration of Zr(Z=40) following the Aufbau principle using the following Fig.
Zr(40)=1s²2s²2p⁶3s²3p⁶4s²3d¹º 4p⁶5s²4d²
But finally the above electron configuration should be written according to value of n as follows.
Zr(40)=1s²2s²2p⁶3s²3p⁶3d¹º 4s²4p⁶4d²5s²
To know alternative method watch this video first and then apply for Zr.
3)Hund's Rule
The distribution of electrons in different degenerate orbitals,i.e,orbitals of the same energy,is governed by Hund's rule.Hund's rule is as follows:
The electrons will distribute themselves in different degenerate orbitals in such a way that maximum number of electrons remain in unpaired state. The spin of the unpaired electrons will be in the same direction.Degenerate orbitals are three p orbitals,five d orbitals and seven f orbitals.Hund's rule is not for s-orbital.
For example:The electron configuration of N(7)=1s²2s²2p³.In reality there are three 2p orbitals of the same energy,which are designated as px ,py ,pz.
According to June's rule these last three electrons will remain in three different p-orbitals with their spin in same direction.So the electron configuration of nitrogen N(7) in box system is as follows:
Similarly,the electron configuration of oxygen O(8)=1s²2s²2p⁴.The electron configuration of oxygen atom in box system is as follows:
Electron Configuration Exceptions
The electron configuration of almost all elements can be confirmed according to Aufbau principle using this Fig.
But there are some exceptions or deviations.Two major reasons behind these deviations or exceptions are as follows:
1)More stability of half filled and full filled orbitals:
It is found that the degenerate orbitals(p,d,f) gain more stability,when they are exactly half filled or full filled.
That means that np³,np⁶,nd⁵,nd¹º,nf⁷and nf¹⁴ configurations are more stable.For this reason the configuration (n-1)d⁵ns¹ becomes more stable than (n-1)d⁴ns¹ and (n-1)d¹ºns¹ becomes more stable than (n-1)d⁹ns².
Therefore the electron configuration of Cr(24) and Cu(29) are as follows:
Cr(24)=
1)More stability of half filled and full filled orbitals:
It is found that the degenerate orbitals(p,d,f) gain more stability,when they are exactly half filled or full filled.
That means that np³,np⁶,nd⁵,nd¹º,nf⁷and nf¹⁴ configurations are more stable.For this reason the configuration (n-1)d⁵ns¹ becomes more stable than (n-1)d⁴ns¹ and (n-1)d¹ºns¹ becomes more stable than (n-1)d⁹ns².
Therefore the electron configuration of Cr(24) and Cu(29) are as follows:
Cr(24)=
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁵4s¹
But not
(1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁴4s²)
Cu(29)=
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹º4s¹
But not
(1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁹4s²)
2)Very small difference of energy between (n+l)d and nf orbitals:
In cases of atoms of higher atomic number,the difference of energy between (n+l)d and nf orbital is very low.Therefore, sometimes one or more electrons may go from nf orbitals to( n+l)d orbitals.
●La(57):
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹º4s²4p⁶4d¹º5s²5p⁶5d¹6s²
In cases of atoms of higher atomic number,the difference of energy between (n+l)d and nf orbital is very low.Therefore, sometimes one or more electrons may go from nf orbitals to( n+l)d orbitals.
●La(57):
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹º4s²4p⁶4d¹º5s²5p⁶5d¹6s²
Although according to general rule,it should have been 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹º4s²4p⁶4d¹º4f¹5s²5p⁶6s²
Related Pages


































Comments
Post a Comment