【】What Is the Phosphorus (P) Electron Configuration?
●Ground State Electron Configuration for phosphorus (P):
1s² 2s² 2p⁶3s² 3p³●Electron Configuration for a Neutral Atom of phosphorus (P):
1s² 2s² 2p⁶3s² 3p³
1s² 2s² 2p⁶3s² 3p³
●Unabbreviated Electron Configuration for phosphorus (P):
1s² 2s² 2p⁶3s² 3p³●Phosphorus Longhand Electron Configuration:1s² 2s² 2p⁶3s² 3p³
●Phosphorus Shorthand Electron Configuration:[Ne]3s² 3p³
●Abbreviated Electron Configuration for phosphorus (P):
[Ne]3s² 3p³
[Ne]3s² 3p³
Explanation:
This process is applicable for all the elements of the periodic table.
Step-2:
(How do you remember the order of electron configurations?)
Now we have to memorize the following sequence of the orbitals.
ss
ps ps
dps dps
fdps fdps fdps fdps....
In a line-
ss ps ps dps dps fdps fdps
fdps fdps fdps fdps.......
Step-3:
Now we have to first number the s-orbital (start with 1s)like this:
1s2s
p3s p 4s
dp 5s dp 6s
fdp7s fdp 8s...... According to your need.
In a line-
1s2s p3s p 4s dp 5s dp 6s
fdp7s fdp 8s........
Remember
● s-orbital start with 1s
● p-orbital start with 2p
● d-orbital start with 3d
● f-orbital start with 4f
Again,Now(second) we need number the p-orbital(start with 2p) like this:
ss
2ps 3ps
d 4ps d 5ps
fd6ps fd7ps fd8ps fd9ps....
In a line--
ss 2ps 3ps d4ps d5ps
fd6ps fd7ps fd8ps fd9ps....
Thirdly,we need number the d-orbital(start with 3d)like this:
ss
ps ps
3dps 4dps
f5dps f6dps f7dps f8dps....
In a line--
ss ps ps 3dps 4dps
f5dps f6dps f7dps f8dps....
Fourthly,number the f-orbital(start with 4f) like this:
ss
ps ps
dps dps
4fdps 5fdps 6fdps 7fdps....
In a line--
ss ps ps dps dps 4fdps 5fdps 6fdps 7fdps......
Fifltly,number s,p,d,f together like this....
1s2s
2p3s 3p4s
3d4p5s 4d5p6s
4f5d6p7s 5f6d7p8s
In a line----
1s 2s 2p 3s 3p 4s 3d 4p 5s d 5p 6s 4f 5d 6p 7s 5f 6d 7p 8s
Step-4:
•S-orbital can take maximum 2 electrons.
•p-orbital can take maximum 6 electrons.
•d-orbital can take maximum 10 electrons.
•f-orbital can take maximum 14 electrons.
Step-5:
Electronic configuration for phosphorus (P) will be...
s² s²
p⁶s² p³s
dps dps
fdps fdps fdps....
So,we are getting..
p⁶s² p³
Now number s-orbitals according to above process.
p⁶ 3s² p³
2p⁶3s² 3p³
Here is the way you can follow to write electron configuration for phosphorus (P) in just 5 steps.
Step-1:
To do electron configuration for phosphorus(P),we have to know the atomic number for phosphorus(P).
The atomic number for phosphorus (P) is 15.So P has 15 electrons and 15 protons.
The atomic number for phosphorus (P) is 15.So P has 15 electrons and 15 protons.
Follow how I find electron configuration for phosphorus(P).
.
.
This process is applicable for all the elements of the periodic table.
Step-2:
(How do you remember the order of electron configurations?)
Now we have to memorize the following sequence of the orbitals.
ss
ps ps
dps dps
fdps fdps fdps fdps....
In a line-
ss ps ps dps dps fdps fdps
fdps fdps fdps fdps.......
Step-3:
Now we have to first number the s-orbital (start with 1s)like this:
1s2s
p3s p 4s
dp 5s dp 6s
fdp7s fdp 8s...... According to your need.
In a line-
1s2s p3s p 4s dp 5s dp 6s
fdp7s fdp 8s........
Remember
● s-orbital start with 1s
● p-orbital start with 2p
● d-orbital start with 3d
● f-orbital start with 4f
Again,Now(second) we need number the p-orbital(start with 2p) like this:
ss
2ps 3ps
d 4ps d 5ps
fd6ps fd7ps fd8ps fd9ps....
In a line--
ss 2ps 3ps d4ps d5ps
fd6ps fd7ps fd8ps fd9ps....
Thirdly,we need number the d-orbital(start with 3d)like this:
ss
ps ps
3dps 4dps
f5dps f6dps f7dps f8dps....
In a line--
ss ps ps 3dps 4dps
f5dps f6dps f7dps f8dps....
Fourthly,number the f-orbital(start with 4f) like this:
ps ps
dps dps
4fdps 5fdps 6fdps 7fdps....
In a line--
ss ps ps dps dps 4fdps 5fdps 6fdps 7fdps......
Fifltly,number s,p,d,f together like this....
1s2s
2p3s 3p4s
3d4p5s 4d5p6s
4f5d6p7s 5f6d7p8s
6f7d8p9s.........
In a line----
1s 2s 2p 3s 3p 4s 3d 4p 5s d 5p 6s 4f 5d 6p 7s 5f 6d 7p 8s
6f 7d 8p 9s.........
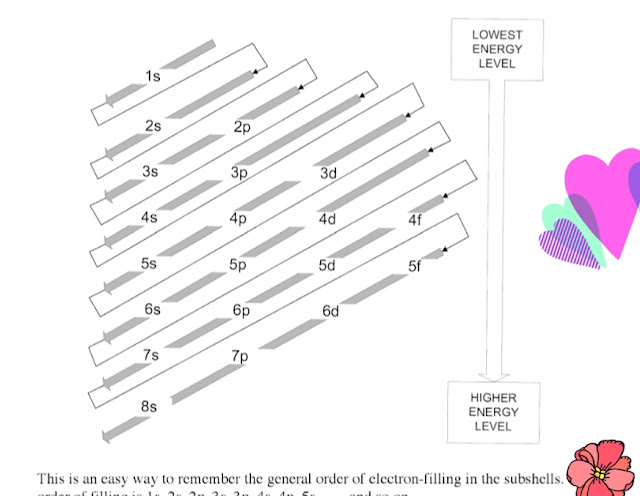 |
How do you remember the order of electron configurations?
|
Step-4:
(What is spdf in electron configuration?
think--)
Now we have to know that
Now we have to know that
•S-orbital can take maximum 2 electrons.
•p-orbital can take maximum 6 electrons.
•d-orbital can take maximum 10 electrons.
•f-orbital can take maximum 14 electrons.
 |
| How to Do or Find Electron Configuration |
Step-5:
Now we know that phosphorus (P) has 15 electrons.
Electronic configuration for phosphorus (P) will be...
s² s²
p⁶s² p³s
dps dps
fdps fdps fdps....
So,we are getting..
s² s²
p⁶s² p³
Now number s-orbitals according to above process.
1s² 2s²
p⁶ 3s² p³
Now number the p orbitals according to above process.
1s² 2s²
2p⁶3s² 3p³
So the electronic configuration for P is
1s² 2s² 2p⁶3s² 3p³
Some Questions and Answers:
●What is the electron configuration for phosphorus(P)?
Ans:
1s² 2s² 2p⁶3s² 3p³
Valence Electron configuration,number of valency electrons and valency shell of phosphorus
3s² 3p³
Valence Electron configuration,number of valency electrons and valency shell of phosphorus
3s² 3p³

















Comments
Post a Comment