【5 Steps】Electron Configuration of Chromium(Cr) in Just 5 Steps||Chromium (Cr)Electron Configuration(Exceptions)
Electron Configuration of Chromium(Cr) in Just 5 Steps
To write electron configuration of chromium (Cr),we will follow just 5 steps.
Step-1:
To write electron configuration of chromium(Cr),we have to know the atomic number of chromium.The atomic number of chromium is 24.So chromium has 24 electrons and 24 protons.
Look how I find the electron configuration of chromium.
Step-2:
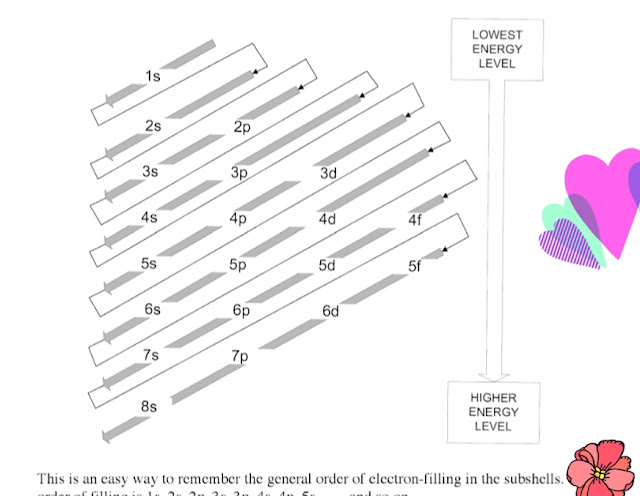
Now we must memorize the following sequence of the orbitals.
ss
ps ps
dps dps
fdps fdps fdps fdps....
In a line-
ss ps ps dps dps fdps fdps
fdps fdps fdps fdps.......
Step-3:
Now we have to first number the s-orbital (start with 1s)like this:
1s2s
p3s p 4s
dp 5s dp 6s
fdp7s fdp 8s...... According to your need.
In a line-
1s2s p3s p 4s dp 5s dp 6s
To write electron configuration of chromium(Cr),we have to know the atomic number of chromium.The atomic number of chromium is 24.So chromium has 24 electrons and 24 protons.
Look how I find the electron configuration of chromium.
Step-2:
Now we must memorize the following sequence of the orbitals.
ss
ps ps
dps dps
fdps fdps fdps fdps....
In a line-
ss ps ps dps dps fdps fdps
fdps fdps fdps fdps.......
Step-3:
Now we have to first number the s-orbital (start with 1s)like this:
1s2s
p3s p 4s
dp 5s dp 6s
fdp7s fdp 8s...... According to your need.
In a line-
1s2s p3s p 4s dp 5s dp 6s
fdp7s fdp 8s........
Remember● s-orbital start with 1s
● p-orbital start with 2p
● d-orbital start with 3d
● f-orbital start with 4f
Again,Now(second) we need number the p-orbital(start with 2p) like this:
ss
2ps 3ps
d 4ps d 5ps
fd6ps fd7ps fd8ps fd9ps....
In a line--
ss 2ps 3ps d4ps d5ps
fd6ps fd7ps fd8ps fd9ps....
Thirdly,we need number the d-orbital(start with 3d)like this:
ss
ps ps
3dps 4dps
f5dps f6dps f7dps f8dps....
In a line--
ss ps ps 3dps 4dps
f5dps f6dps f7dps f8dps....
Fourthly,number the f-orbital(start with 4f) like this:
ss
ps ps
dps dps
4fdps 5fdps 6fdps 7fdps....
In a line--
ss ps ps dps dps 4fdps 5fdps 6fdps 7fdps......
Finally,number s,p,d,f together like this....
1s2s
2p3s 3p4s
3d4p5s 4d5p6s
4f5d6p7s 5f6d7p8s
6f7d8p9s.........
In a line----
1s 2s 2p 3s 3p 4s 3d 4p 5s d 5p 6s 4f 5d 6p 7s 5f 6d 7p 8s
6f 7d 8p 9s.........
How to Do Electron Configuration for Cr
Step-4:
Now we have to know that
•S-orbital can take maximum 2 electrons.
◆p-orbital can take maximum 6 electrons.
•d-orbital can take maximum 10 electrons.•f-orbital can take maximum 14 electrons.
 |
| How to Do or Find Electron Configuration of Cr |
Step-5:
Now we know that chromium has 24 electrons.
Electron configuration of Chromium will be...
s² s²
d⁴
d⁴
Electron configuration of Chromium will be...
s² s²
p⁶s² p⁶s²
d⁴ps dps
fdps fdps fdps....
So,we are getting..
s² s²
p⁶s² p⁶s²
d⁴
Now we need to number s-orbital according to above process.
1s² 2s²
p⁶3s² p⁶4s²
p⁶3s² p⁶4s²
d⁴
Now number p-orbital according to above process.
1s² 2s²
2p⁶3s² 3p⁶4s²
d⁴
Now number d-orbital according to above process
1s² 2s²
2p⁶3s² 3p⁶4s²
3d⁴
In a line....
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁴
This should be the electronic configuration but unfortunately this is not the electronic configuration of chromium.Chromium has exceptional electronic configuration.
●we have to bear in mind that half-filled and full filled (p,d,f) orbitals gain more stability.That means that np³,np⁶,nd⁵,nd¹º,nf⁷ and nf¹⁴ configurations are more stable.
In a line.......
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁴
From above,we see that 4s has 2 electrons and immediately before 4s,we see 3d has 4 electrons.So 3d⁴ is not stable.It(3d) can be easily stable by taking one electron from 4s.Remember (n-1)d⁵ns¹ is
more stable than(n-1)d⁴ns²
●so, 4s² will be 4s¹
and 3d⁴ will be 3d⁵
So the electronic configuration of chromium is given below:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d⁵
●We know that the electronic configuration of any element is written in ascending order(low to high) of n.So.....
1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁵4s¹
Full Electronic Configuration of chromium:
















Comments
Post a Comment