【】What is the formula and charge of ammonium ion?
The formula for ammonium ion is NH₄⁺.
Explanation:
Today I will show you the formula for ammonium in just two steps.
Step-1:
To write the formula for ammonium,we have to know the formula for ammonia.
NH₃ is the chemical formula for ammonia.
Step-2:
In ammonia,nitrogen has one lone pair.As a result,ammonia can accept one H⁺ and we get the formula for ammonium.
NH₃ +H⁺ = NH₄⁺
Today I will show you the formula for ammonium in just two steps.
Step-1:
To write the formula for ammonium,we have to know the formula for ammonia.
NH₃ is the chemical formula for ammonia.
Step-2:
In ammonia,nitrogen has one lone pair.As a result,ammonia can accept one H⁺ and we get the formula for ammonium.
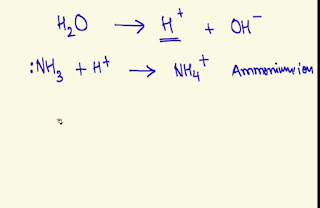 |
Formula for Ammonium
|
NH₃ +H⁺ = NH₄⁺
NH₄⁺ is the formula for ammonium.Ammonium is a polyatomic cation.It has +1 charge and it's shape is tetrahedral.









how do u calculate the volume of 1 mole of nitrogen at rtp and stp
ReplyDelete