【】CO (Carbon Monoxide) Lewis Dot Structure with Formal Charge
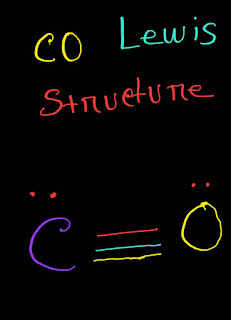
CO (Carbon Monoxide) Lewis Structure Answer : The definition of carbon monoxide (CO) Lewis structure is given below: CO Lewis structure(carbon monoxide electron dot structure) is that type of structure where we show the total ten valence electrons of CO as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots(●●) but a lone pair of two electrons is shown by dots[●●]. In CO Lewis structure,the carbon atom follows the octet rule and the oxygen atom also follows the octet rule.So,CO follows the octet rule for all the atoms. In CO Lewis structure,we get five pairs of electrons.Out of five pairs of electrons,CO has three bond pairs and two lone pairs of electrons.Here carbon has one lone pair and oxygen has one lone pair of electrons. Explanation: Hello,today I am going to show you the CO Lewis structure in just few easy steps. CO Lewis Structure Setup Step-01: To draw the CO Lewis struct

