【 】How Many Neutrons Does Zirconium(Zr) Have?||Number of Neutrons in Zirconium (Zr)
How Many Neutrons Does Zirconium(Zr) Have?
Answer:
zirconium(Zr) has 51 neutrons for this isotope.
Explanation:
Hello, chemistry lover! today we are going to help you to find out how many neutrons zirconium(Zr)have in just 3 steps.
Explanation:
Hello, chemistry lover! today we are going to help you to find out how many neutrons zirconium(Zr)have in just 3 steps.
Description
Description
Descriptio
To find out the number of neutrons for zirconium(Zr) ,we have to have knowledge about atomic number,atomic mass,mass number of zirconium(Zr)
.Click picture to see clearly.
Step-1:
●The atomic number of zirconium(Zr) is the number of protons in zirconium(Zr)'s nucleus.
●The atomic mass of zirconium(Zr) is the average number of protons plus neutrons of all the isotopes of zirconium(Zr) .
●The masss number of zirconium(Zr) is the number of protons plus neutrons of a specific isotope of zirconium(Zr) .
Step-2:
We need to convert atomic mass of zirconium(Zr) to mass number of zirconium(Zr) by rounding if necessary (In case of fraction) .
From periodic table,
●Atomic number of zirconium(Zr) is 40.
●Atomic mass of zirconium(Zr) is 91.22
●To find out mass number,we need to round 91.22 to a whole number.
So,by rounding,we are getting mass number of zirconium(Zr).The mass
number of zirconium(Zr) is 91.
Step-3:
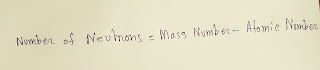
By the difference between mass number and atomic number of zirconium(Zr),we get the number of neutrons in zirconium(Zr).
●Number of neutrons=91-40=51
In another way,
●Mass number=Protons +Neutrons
Or,91 =40+Neutrons
Or,91-40=Neutrons
Therefore,
Neutrons=51
Therefore,the number of neutrons in zirconium(Zr) is 51 here for this isotope.Remember that neutrons are present in the nucleous of zirconium(Zr)and it's charge is zero.
●Number of protons in zirconium(Zr) is 40.Protons are present in the nucleus of zirconium(Zr) .
● Number of electrons in zirconium(Zr) is 40.Remember that number of electron equals to proton in a neutral atom.Eletrons are present outside of nucleus of zirconium(Zr) .
Continue
.Click picture to see clearly.
Step-1:
●The atomic number of zirconium(Zr) is the number of protons in zirconium(Zr)'s nucleus.
●The atomic mass of zirconium(Zr) is the average number of protons plus neutrons of all the isotopes of zirconium(Zr) .
●The masss number of zirconium(Zr) is the number of protons plus neutrons of a specific isotope of zirconium(Zr) .
Step-2:
We need to convert atomic mass of zirconium(Zr) to mass number of zirconium(Zr) by rounding if necessary (In case of fraction) .
From periodic table,
●Atomic number of zirconium(Zr) is 40.
●Atomic mass of zirconium(Zr) is 91.22
●To find out mass number,we need to round 91.22 to a whole number.
So,by rounding,we are getting mass number of zirconium(Zr).The mass
number of zirconium(Zr) is 91.
Step-3:
Step-3:
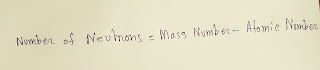
By the difference between mass number and atomic number of zirconium(Zr),we get the number of neutrons in zirconium(Zr).
●Number of neutrons=91-40=51
In another way,
●Mass number=Protons +Neutrons
Or,91 =40+Neutrons
Or,91-40=Neutrons
Therefore,
Neutrons=51
Therefore,the number of neutrons in zirconium(Zr) is 51 here for this isotope.Remember that neutrons are present in the nucleous of zirconium(Zr)and it's charge is zero.
●Number of protons in zirconium(Zr) is 40.Protons are present in the nucleus of zirconium(Zr) .
● Number of electrons in zirconium(Zr) is 40.Remember that number of electron equals to proton in a neutral atom.Eletrons are present outside of nucleus of zirconium(Zr) .
Continue










Comments
Post a Comment