【 】How Many Protons Does Lithium Have?||Number of Protons in Lithium||Atomic Number of Lithium
How Many Protons Does Lithium Have?
Answer:
Lithium has three protons.
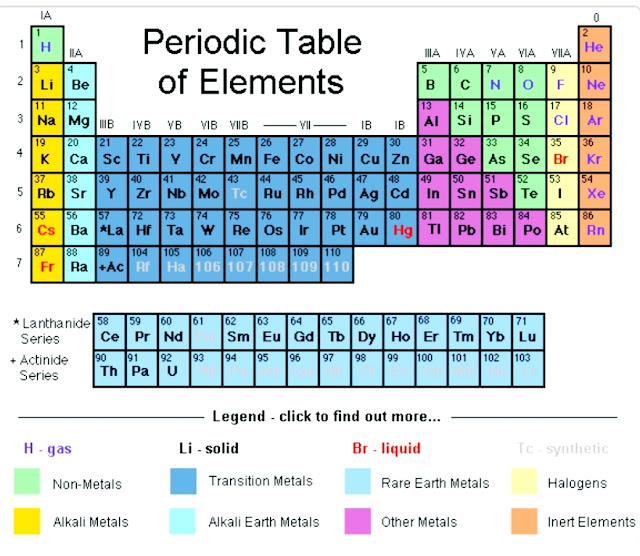
From the periodic table,we see that the symbol for lithium is Li.Look at IA group .
Step-2:
Now we will look at the number situated upper right of the lithium element's symbol.
From periodic table ,we observe that this number for lithium is 3.This number is called atomic number and expresses the number of protons in the lithium's element.So there are three protons in lithium.
First,we have to look for the symbol for lithium in the periodic table.
Step-2:
Now we will look at the number situated upper right of the lithium element's symbol.
From periodic table ,we observe that this number for lithium is 3.This number is called atomic number and expresses the number of protons in the lithium's element.So there are three protons in lithium.
Nice to know:
Remember that the atomic number of lithium is 3.This means that in a neutral lithium atom we have three protons and three electrons.
As the charge of proton is +1 and the charge of electron is -1,equal number of protons and electrons make a lithium atom neutral or chargeless
Continue
As the charge of proton is +1 and the charge of electron is -1,equal number of protons and electrons make a lithium atom neutral or chargeless
Continue







Comments
Post a Comment