【】How Many Valence Electrons Does Nitrogen (N ) Have?
How Many Valence Electrons Does Nitrogen Have?
Answer:
Nitrogen has five valence electrons.
Explanation:
Now I am going to show you how many valence electrons nitrogen have in just 5 steps.
Step-1:
First, find the atomic number of nitrogen from periodic table.
From periodic table ,we see that the atomic number of nitrogen is 7.
Step-2:
We know that the atomic number of nitrogen is 7.So nitrogen has 7 protons and 7 electrons as the charge of electrons and protons are equal but opposite in nature.The charge of proton is +1 and the charge of electron is -1.
Step-3:
N(7)=1s²2s²2p³
Step-3:
Now write the electron configuration of nitrogen.
N(7)=1s²2s²2p³
Step-4:
N(7)=1s²2s²2p³
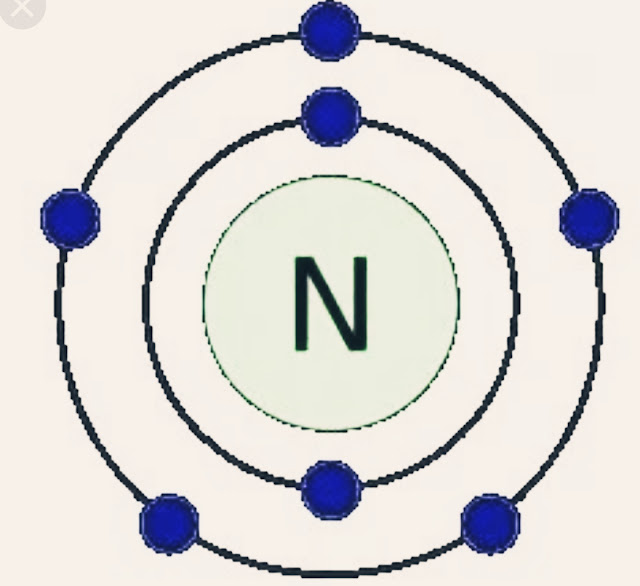
Now we will find out valence shell of nitrogen from the electronic configuration of nitrogen.
N(7)=1s²2s²2p³
Valence shell can be found from the highest number of principal quantum number.You know that principal quantum number is expressed by n.
N(7)=1s²2s²2p⁵
Here the highest value of n is 2.SoThe valence shell of nitrogen is 2s²2p³.
Step-5
In this step,we will find out the valence electrons of nitrogen.We have to know that the electrons of valence shell are called valence electrons.
N(7)=1s²2s²2p³
N(7)=1s²2s²2p³
From the above electron configuration of nitrogen,we see that nitrogen has 5 valence electrons in its valence shell.









A nitrogen atom is the simplest atom of all that has only one valence electron. But it has so many electrons in it that it can be said to have a total of six. This adds up to 18 electrons and makes nitrogen a highly electronegative element.
ReplyDeleteIt's used as the first "molecule" in many compounds like ammonia and nitrite. But Nitrogen also exists in different forms, e.g., as a gas or an ion (eutectic mixture). There are so many different types of nitrogen that scientists haven't been able to identify them yet. An example is called the element's 22-gonatonic acid (N2O4) which has 6 protons and 3 neutrons but is chemically inactive since it doesn't react with anything at all.