【●3 Steps】How Many Electrons Does Oxygen(O) Have?||Number of Electrons in Oxygen(O)
How Many Electrons Does Oxygen(O) Have?||Number of Electrons in Oxygen(O)
Now I would like to show you how many electrons oxygen have in just three steps.
Step-1:
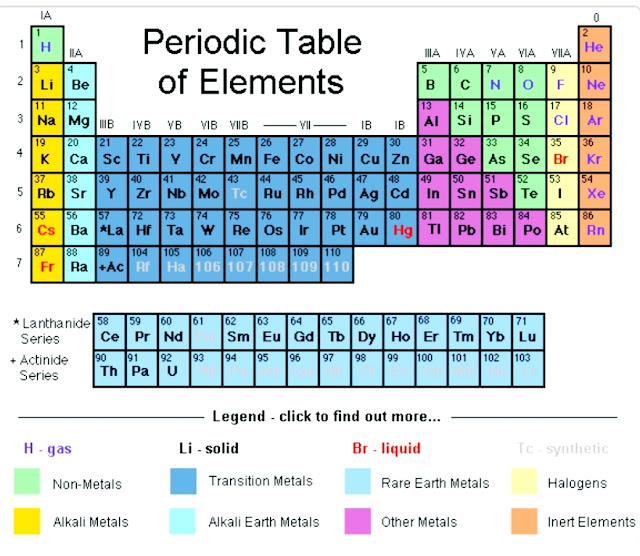
First,we need to look for the symbol for oxygen in the periodic table.
Step-2:
From periodic table ,we observe that the atomic number of oxygen is 8.
Step-3:
We know that the atomic number of oxygen is 8.This means that we have 8 protons. The charge of proton is +1.On the other hand, the charge of electron is -1.
So for neutral oxygen , we have 8 negative charges which are electrons.
Therefore,the number of electrons in oxygen is 8.
Step-4:
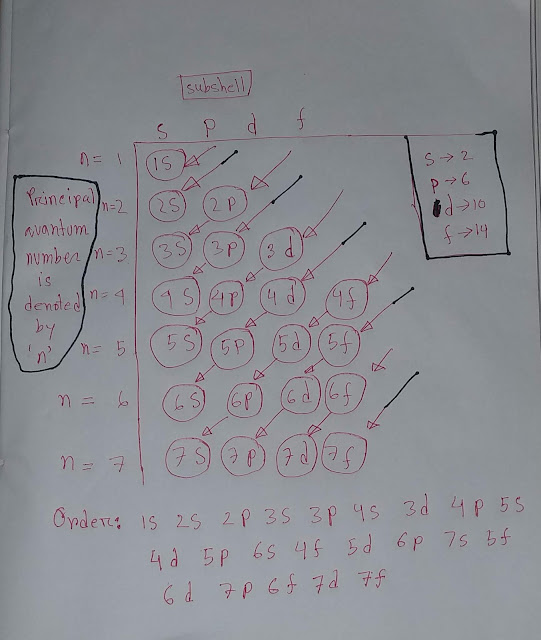
Now we do electronic configuration of oxygen .
O(8)=1s²2s²2p⁴
💝In oxygen
◆1s subshell holds two electrons
◆2s subshell holds two electrons
◆2p subshell holds four electrons
Totally,we are getting eight electrons in oxygen.
Continue








Comments
Post a Comment