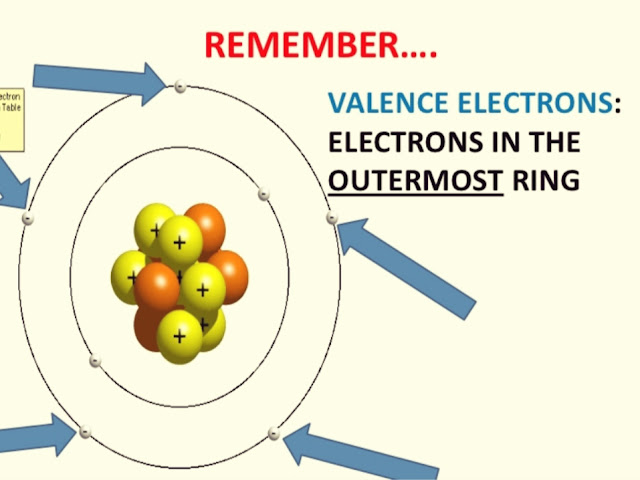
【】How Many Valence Electrons Does Aluminum Have?||# of valence electrons in aluminum
Answer:
Aluminum has three valence electrons.
Explanation:
Step-3:
Al(13)=1s²2s²2p⁶3s²3p¹3dº
Step-4:
Now we will find out valence shell of aluminum from the electronic configuration of aluminum.
Al(13)=1s²2s²2p⁶3s²3p¹3dº
Al(13)=1s²2s²2p⁶3s²3p¹ 3dº
Here the highest value of n is 3.SoThe valence shell of aluminum is 3s²3p¹3dº.
Step-5
Al(13)=1s²2s²2p⁶3s²3p¹
configuration
of aluminum,we see
that aluminum has 3
valence electrons in its
valence shell(3s²3p¹).
Now I am going to show you how many valence electrons aluminum have in just 5 steps.
Step-1:
First, find the atomic number of aluminum from periodic table.
From periodic table ,we see that the atomic number of aluminum is 13.
Step-2:
We know that the atomic number of aluminum is 13.So aluminum has 13 protons and 13 electrons as the charge of electrons and protons are equal but opposite in nature.The charge of proton is +1 and the charge of electron is -1.
Step-3:
Now write the electron configuration of aluminum .
Al(13)=1s²2s²2p⁶3s²3p¹3dº
Step-4:
Al(13)=1s²2s²2p⁶3s²3p¹3dº
Now we will find out valence shell of aluminum from the electronic configuration of aluminum.
Al(13)=1s²2s²2p⁶3s²3p¹3dº
Valence shell can be found from the highest number of principal quantum number.
You know that principal quantum number is expressed by n.
You know that principal quantum number is expressed by n.
Al(13)=1s²2s²2p⁶3s²3p¹ 3dº
【Note:
The ‘l’ value for valency
electrons in ‘Al’ is ?
Answer:
l -->>Angular momentum quantum number
For n =3 ,the value for "l" is 0,1,2.
For "l" = 0 ,we get s subshell.
For "l" =1 ,we get p subshell.
For "l " =2 'we get d subshell
As there is no electrons in 3d,so the l value for valence electrons in Al is 0 and 1.
Again,the 0 value of "l" indicate
"s " subshell and the 1 value of "l" indicates p subshell.
●The valence shell of aluminum is 3s²3p¹
】
Step-5
In this step,we will find out the valence electrons of aluminum.
We have to know that the electrons of valence shell are called valence electrons.
Al(13)=1s²2s²2p⁶3s²3p¹
From the above electron
configuration
of aluminum,we see
that aluminum has 3
valence electrons in its
valence shell(3s²3p¹).
So there are three valence electrons of aluminum.

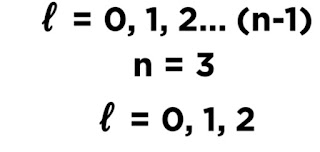








Comments
Post a Comment