【】How Many Electrons Does Carbon(C) Have?
Answer:
Carbon has six electrons.
Explanation:
Now I would like to show you how many electrons a carbon has in just three steps.
Step-1:
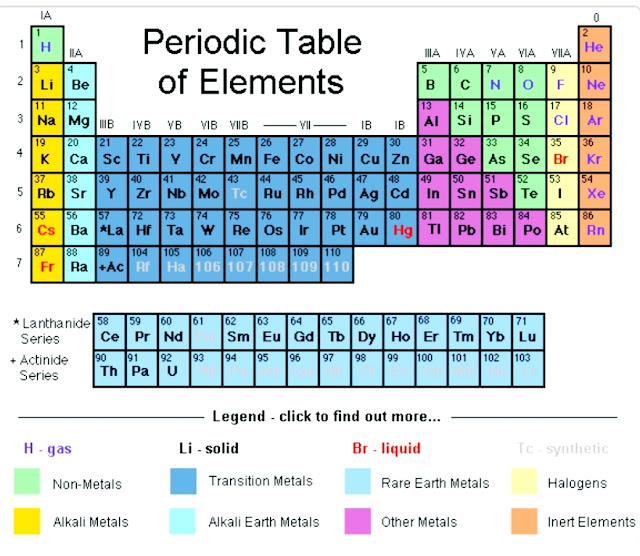
First,we need to look for the symbol for carbon in the periodic table.
Step-2:
From periodic table ,we observe that the atomic number of carbon is 6.
Step-3:
We know that the atomic number of carbon is 6.This means that we have 6 protons. The charge of proton is +1.On the other hand, the charge of electron is -1.
So for neutral carbon, we have six negative charges which are electrons.
Therefore,the number of electrons in carbon is 6.
So for neutral carbon, we have six negative charges which are electrons.
Therefore,the number of electrons in carbon is 6.
Step-4:
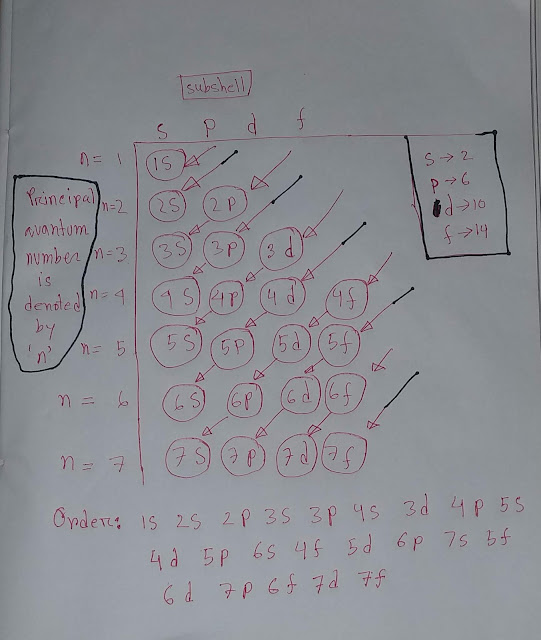
Now we do electron configuration of carbon .
C(6)=1s²2s²2p²
💝In carbon
◆1s subshell holds two electrons
◆2s subshell holds two electrons
◆2p subshell holds two electrons
Totally,we are getting six electrons in carbon.








Comments
Post a Comment