【】What is the carbonate formula?
The formula for carbonate is CO₃2- .
The charge of carbonate polyatomic ion is -2.
In carbonate,one oxygen is bonded to carbon with double bond and two other oxygens are bonded to carbon with single bond.So Structural formula for carbonate is given below:
By using carbonate anion formula CO₃2-
,we get the formula for the following compounds.:
1)Ferric carbonate formula
Fe₂(CO₃)₃
2)Carbonic acid formula
H₂CO₃
3)Zinc carbonate formula
ZnCO₃
4)Lead carbonate formula
PbCO₃
5)Sodium carbonate formula
Na₂CO₃
6)Calcium carbonate formula
CaCO₃
7)Aluminum carbonate formula
Al₂(CO₃)₃
8)Nickel carbonate formula
NiCO₃
Continue
The charge of carbonate polyatomic ion is -2.
Explanation:
Today I will show you the formula for carbonate in this blog post.
Carbonate is a polyatomic ion.It has -2 charge.In carbonate,we get one carbon and three oxygens.
So the formula for carbonate is CO₃2-
In carbonate ion,the oxidation number of carbon is +4 and the oxidation number of O is -2.The net charge of carbonate is -2.
Carbonate is a polyatomic ion.It has -2 charge.In carbonate,we get one carbon and three oxygens.
So the formula for carbonate is CO₃2-
 |
Formula for Carbonate |
In carbonate ion,the oxidation number of carbon is +4 and the oxidation number of O is -2.The net charge of carbonate is -2.
In carbonate,one oxygen is bonded to carbon with double bond and two other oxygens are bonded to carbon with single bond.So Structural formula for carbonate is given below:
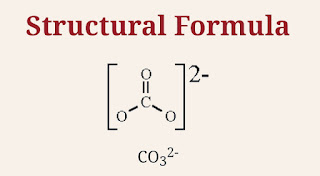 |
| Structural formula for Carbonate ion |
By using carbonate anion formula CO₃2-
,we get the formula for the following compounds.:
1)Ferric carbonate formula
Fe₂(CO₃)₃
2)Carbonic acid formula
H₂CO₃
3)Zinc carbonate formula
ZnCO₃
4)Lead carbonate formula
PbCO₃
5)Sodium carbonate formula
Na₂CO₃
6)Calcium carbonate formula
CaCO₃
7)Aluminum carbonate formula
Al₂(CO₃)₃
8)Nickel carbonate formula
NiCO₃
Continue









Comments
Post a Comment