【】What is the Ferric Carbonate Formula?
Fe₂(CO₃)₃ is the ferric carbonate formula.
Explanation:
Hello!everyone,today I am going to show you ferric carbonate formula.
Step-1:We know that ferric means Fe³+.That is the oxidation number of iron will be 3+.
Step-2:Now we need to know the formula of carbonate.The formula for carbonate is
CO₃2-
Step-3:Now place the two ion side by side.
Fe³+ CO₃2-
Step-2:Now we need to know the formula of carbonate.The formula for carbonate is
CO₃2-
Step-3:Now place the two ion side by side.
Fe³+ CO₃2-
Step-4:Now put the superscript of carbonate as the subscript of ferric and put the supperscript of ferric as the subscript of carbonate.This is called Criss-cross method.
So the ferric carbonate formula is given below:
Fe₂(CO₃)₃
Step-5:Now watch this video.
Continue


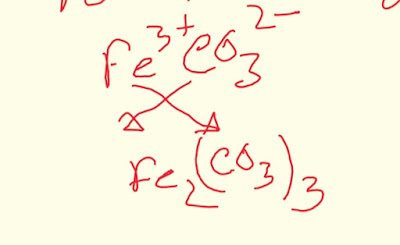







Comments
Post a Comment