【】What is the Chemical Formula for Acetate Ion
The formula of acetate is CH₃COO-.The charge of acetate is -1.
Step-1:
The acetate formula is CH₃COO-.The charge of acetate is -1.
Step-2:
CH3COOH
Step-3:Common System of Nomenclature
Many monocarboxylic acids have been extracted from natural sources.
And these acids have been named according to the Latin name of the sources by adding 'ic acid' to it.
●Acetic acid---- CH3COOH--Source--Latin,Acetum.
Now minus 'um' from 'acetum' and add ic acid.So we are getting acetic acid(CH3COOH).
If one hydrogen is deducted from acetic acid, then we get
CH₃COO- acetate ion.
Step-4:
In IUPAC system,the carboxylic acid is called alkanoic acid(R-COOH).In this system,in case of mono carboxylic acid,the 'e' from the name of the alkane is replaced by 'oic acid'.The name of the alkane is selected by finding the longest carbon chain.
●RCOOH---alkanoic acid:【Alkane-e +oic acid =Alkanoic acid】
●CH3COOH------ethanoic acid
【Ethane-e +oic acid =Ethanoic acid】
If you minus one hydrogen from ethanoic acid(CH3COOH) ,you will get ethanoate ion(Ethanoic acid -ic acid plus ate =Ethanoate)
The IUPAC name for acetate formula is ethanoate ion.
To get name ethanoate ion ,you just have to minus ic acid and add' ate 'from ethanoic acid.
The formula for acetate ion is given below:
CH₃COO-
1 2
The number one carbon is sp³ hybridized and the second carbon is sp² hybridized.
Nice to Know:
By using acetate formula,we get the formula of following compound.
1)Sodium acetate formula
CH₃COONa
2) Potassium acetate formula
CH₃COOK
3)Calcium acetate formula
( CH₃COO)₂Ca
4)Ammonium acetate formula
CH₃COONH₄
5) Lead acetate formula
(CH₃COO)₂pb
Continue
Explanation:
Today in this post I will show you acetate formula.I will also show you how get acetate formula and it's name.SO let's get started.
Step-1:
The acetate formula is CH₃COO-.The charge of acetate is -1.
Step-2:
The general formula for alkane is CnH2n+2.But the general formula of carboxylic acid is R-COOH.Here -COOH group is common for all carboxylic acid.
Here R- means alkyl group.The general formula for alky group(R-)is CnH2n+1. So any carboxylic acid can be written as CnH2n+1 -COOH.
In case of acetic acid ,we have a alkyl group whose carbon is one.So the acetic acid formula will be the following thing.
CH3COOH
Step-3:Common System of Nomenclature
Many monocarboxylic acids have been extracted from natural sources.
And these acids have been named according to the Latin name of the sources by adding 'ic acid' to it.
●Acetic acid---- CH3COOH--Source--Latin,Acetum.
Now minus 'um' from 'acetum' and add ic acid.So we are getting acetic acid(CH3COOH).
If one hydrogen is deducted from acetic acid, then we get
CH₃COO- acetate ion.
Step-4:
In IUPAC system,the carboxylic acid is called alkanoic acid(R-COOH).In this system,in case of mono carboxylic acid,the 'e' from the name of the alkane is replaced by 'oic acid'.The name of the alkane is selected by finding the longest carbon chain.
●RCOOH---alkanoic acid:【Alkane-e +oic acid =Alkanoic acid】
●CH3COOH------ethanoic acid
【Ethane-e +oic acid =Ethanoic acid】
If you minus one hydrogen from ethanoic acid(CH3COOH) ,you will get ethanoate ion(Ethanoic acid -ic acid plus ate =Ethanoate)
The IUPAC name for acetate formula is ethanoate ion.
To get name ethanoate ion ,you just have to minus ic acid and add' ate 'from ethanoic acid.
Step-5:
The structural formula of acetate ion is given below:
Step-6:
The Condensed structural formula of acetate ion is given below:
CH₃COO-
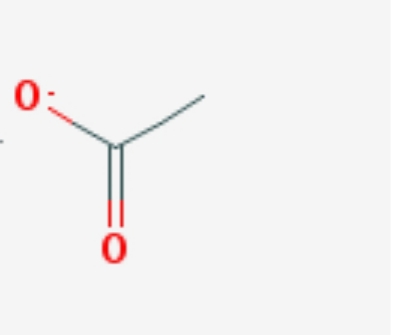
Step-7
The bond line formula of acetate is given below:
Acetate Formula and Hybridization
The formula for acetate ion is given below:
CH₃COO-
1 2
The number one carbon is sp³ hybridized and the second carbon is sp² hybridized.
Nice to Know:
By using acetate formula,we get the formula of following compound.
1)Sodium acetate formula
CH₃COONa
2) Potassium acetate formula
CH₃COOK
3)Calcium acetate formula
( CH₃COO)₂Ca
4)Ammonium acetate formula
CH₃COONH₄
5) Lead acetate formula
(CH₃COO)₂pb
Continue









Comments
Post a Comment