【】Chemical Formula for Sodium Chloride||Sodium Chloride Formula
Chemical Formula for Sodium Chloride
Hello!In this blog post,I am going to show you the chemical formula for sodium chloride in some steps.
Step-1;
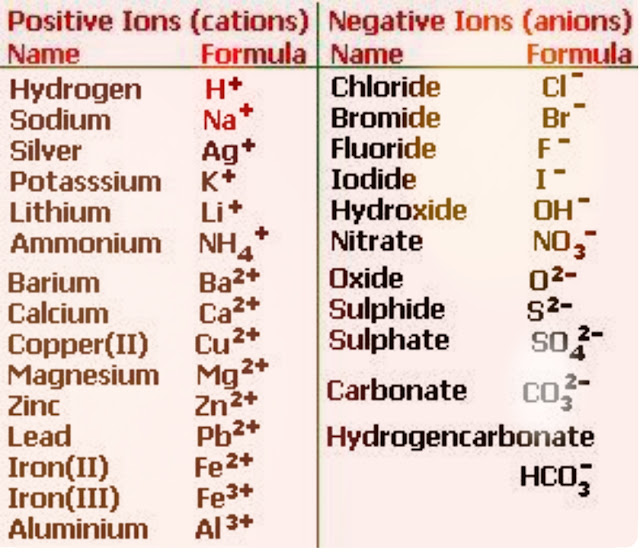
Sodium chloride is a salt.It is composed of two ions.One is sodium cation and the other one is chloride anion.
Step-2:
The formula for sodium ion is Na+ and the formula for chloride ion is Cl-
-.
-.
Step-3:
Now we have to balance the charge of sodium and chloride ion.The charge of sodium is +1 and the charge of chloride is -1
Na+ Cl-
Here positive and negative charge is equal.So we have nothing to do.
Step-4:
So,the chemical formula of sodium chloride is given below:








Comments
Post a Comment