【7 and 5】How to Convert Methane to Ethane and Ethane to Methane
Methane to Ethane Conversion

Methane to Ethane Conversion
Step-1:
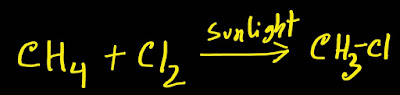
Methane reacts with chlorine in presence of sunlight and produce chloromethane(CH₃-Cl).
Step-2:
Chloromethane reacts with aquous NaOH and produce methanol (CH₃-OH).
Methanol reacts with phosphorus triiodide(PI₃ )and produce iodomethane(CH₃-I).
Iodomethane(CH₃-I) reacts with KCN (alcoholic) and produce ethanenitrile(CH₃-CN).
Step-5:Ethanenitrile(CH₃-CN) is reduced by sodium(Na) and ethanol(CH₃-CH₂-OH) and we get ethanamine or ethyl amine(CH₃-CH₂-NH₂).
Step-6:Ethanamine or ethyl amine(CH₃-CH₂-NH₂) reacts with NOCl and produce chloroethane(CH₃-CH₂-Cl).
Step-7:Chloroethane(CH₃-CH₂-Cl) reacts with Zn-Cu +CH3OH and produce ethane(CH₃-CH₃).

Ethane to Methane Conversion
Ethane to Methane Conversion
Step-1:Ethane(CH₃-CH₃) reacts with chlorine in presence of sunlight and produce chloroethane(CH₃-CH₂-Cl).
Step-2:Chloroethane(CH₃-CH₂-Cl) reacts with aqueous KOH and produce ethanol(CH₃-CH₂-OH).
Step-3:Ethanol(CH₃-CH₂-OH) is oxidized by potassium dichromate (K₂Cr₂O₇) and sulphuric acid and we get ehanal(CH₃-CHO).
Step-4:Ehanal(CH₃-CHO) is also oxidized by potassium dichromate (K₂Cr₂O₇) and Sulphuric acid (H₂SO₄) and we get ethanoic acid(CH₃-COOH).
Step-5:Ethanoic acid(CH₃-COOH) is heated with sodalime (NaOH+CaO).Thus one molecule CO₂ goes out and we get methane(CH₄).
Next
Step-2:Chloroethane(CH₃-CH₂-Cl) reacts with aqueous KOH and produce ethanol(CH₃-CH₂-OH).
Step-3:Ethanol(CH₃-CH₂-OH) is oxidized by potassium dichromate (K₂Cr₂O₇) and sulphuric acid and we get ehanal(CH₃-CHO).
Step-4:Ehanal(CH₃-CHO) is also oxidized by potassium dichromate (K₂Cr₂O₇) and Sulphuric acid (H₂SO₄) and we get ethanoic acid(CH₃-COOH).
Step-5:Ethanoic acid(CH₃-COOH) is heated with sodalime (NaOH+CaO).Thus one molecule CO₂ goes out and we get methane(CH₄).
Next











Comments
Post a Comment