How to Draw CO2 Lewis Structure
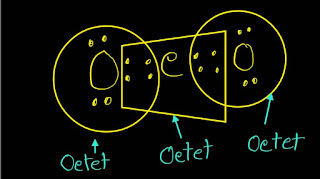
In CO2 Lewis structure,there are 16 valence electrons totally.Carbon has four valence electrons and each oxygen has six valence electrons.So,carbon will form four bonds and oxygen will form two bonds to satisfy the octet rule.
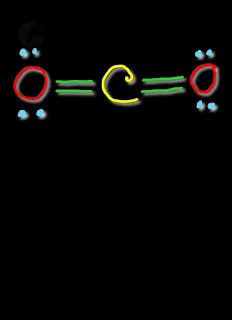 |
| CO2 Lewis Structure |
In CO2 Lewis structure,carbon has four bonds,two with each oxygen.There is no lone pairs electrons of carbon but each oxygen has two lone pairs of electrons.Totally,we get four lone pairs of electrons.
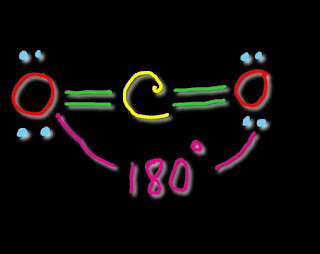 |
| CO2 Molecular Geometry |
In CO2 Lewis structure,we get two sigma bond and two pi bonds.To determine hybridization of carbon,we will consider the steric number or electron regions around carbon.We get two electron regions ignoring pi bonds of carbon.So carbon is sp hybridized.As there is no lone pairs over carbon,the molecular geometry of CO2 will be linear.Thus,the bond angle O--C--O is 180 degree.
Due to linear shape of CO2 ,the net molecular dipole moment of CO2 is zero.This explains that CO2 is a nonpolar molecule.
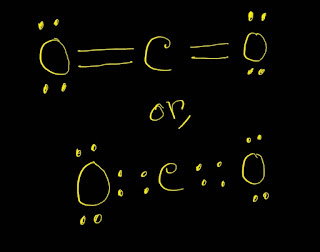
CO2 Lewis Structure Setup Guidelines
1.Place the carbon atom between the two oxygen atoms.
2.Now draw four bonds.Two will go with each oxygen atom.
3.After that,place two lone pairs with each oxygens.Thus you get the best CO2 Lewis structure.
Properties of CO2
Due to linear shape,CO2 is a symmetric nonpolar molecule.That is why it tends to be a gas.
CO2 has very low boiling point which is around -80 ℃ or -100 ℉.It can be liquified and even solidified that produce dry ice.
CO2 can contribute to global warming.Due to bending vibration,CO2 absorbs IR radiation and can contribute to global warming.








Comments
Post a Comment