Does CO2(Carbon dioxide) follow/obey the octet rule?
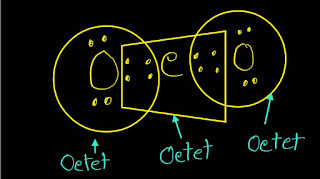
Yes,in the best Lewis structure of CO2(carbon dioxide),the carbon atom,C,and O atom follow or obey the octet rule.
Explanation:
The carbon atom has 4 electrons in outermost shell or valence shell as it is the element of IVA. It shares 4 of its electrons with 2 oxygen atoms(two electrons with each O) and thus it has no lone
pairs of electrons.
Thus 4 its and 4 that of two oxygens completes its octet. While the two oxygens(O) complete their octet.
In more explanation,I will say that carbon is a nonmetal and oxygen is also a nonmetal.So,both atoms in CO2 will share electrons in such a way that both atoms can achieve eight valence electrons in their valence shells and thus can gain more stability.
In CO2 ,each oxygen has shared two electrons with C and C has shared four electrons with two oxygen atoms.
By sharing ,all atoms -C,O and O - in CO2, has achieved the eight valence electrons in their most occupied energy levels,so CO2 follows or obeys the octet rule .








Comments
Post a Comment