【 】SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
SO2 Lewis Structure
Answer:Here is the definition of SO2 Lewis structure.
SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots(●●) but a lone pair of two electrons is shown by dots[●●].
In SO2 Lewis structure,there are totally five lone pairs of electrons.The each oxygen has two lone pairs of electrons and the sulphur atom has one lone pair of electrons.
In SO2 Lewis structure,the oxygen atoms follow the octet rule but the sulfur atom don't follow the octet rule.It has more than eight electrons.
In SO2 Lewis structure,we get nine pairs of electrons.Out of nine pairs of electrons,SO2 has four bond pairs of electrons and five lone pairs of electrons.Here,each oxygen carries two lone pair and the sulfur atom carries one lone pair.
Explanation:
Hello,today I am going to show you how to draw the SO2 Lewis structure in just five steps.
SO2 Lewis Structure Setup Step-1:
To draw the SO2 Lewis structure, we have to find out the SO2 valence electrons first.We express valence electrons as dots in lewis dot structure.
To get the valence electrons of sulfur,we need to look at the electronic configuration of sulfur.
S(16)=1s²2s²2p⁶ 3s² 3p⁴ 3d
The highest value of principal quantum number here is n=3.
The highest value of principal quantum number ,n , indicates the valence shell and we know the electrons in valence shell is called valence shell.

The nunber of valence electrons in sulfur is 6.
Now,to draw the lewis dot structure of sulfur,we need to imagine a square around sulfur.
We know a square has four sides.At first,we have to place one valence electrons as dot to every side of that square before pairing up.
Again, we have to find out the valence electrons of oxygen.
To get the valence electrons of oxygen,we need to look at the electronic configuration of oxygen.
O(8)=1s²2s²2p⁴
The highest value of principal quantum number here is n=2.
The highest value of principal quantum number ,n , indicates the valence shell and we know the electrons in valence shell is called valence shell.

The nunber of valence electrons in oxygen is 6.
Now to draw the lewis dot structure of oxygen,we need to imagine a square around oxygen.

We know a square has four sides.At first,we have to place one valence electrons as dot to every side of that square before pairing up.

SO2 Lewis Structure Setup Step-2: SO2 Valence Electrons
Now we have to count total number of SO2 valence electrons.
S = 6
2×O=6×2=12
So, the total number of valence electrons for SO2 molecule is eighteen ( 6+12= 18) .
Here is the another way to find out SO2 valence electrons.
Sulfur is the element of group 6A,so it has six valence electrons.Besides,the oxygen is the element of group 6A ,so it has six valence electrons.
For SO2 molecule,
S --> 6
2O -->6 ×2 =12
-----------------------
SO2 = 18 Valence Electrons
SO2 Lewis Structure Setup Step-3:
Now we have to determine the central atom in SO2.The central atom is that kind of atom that is single or that has lower electronegativity.In case of SO2, S is the central atom and oxygen ,O, is the outer atom as sulfur is less electronegative than than O.
SO2 Lewis Structure Setup Step-4:
Now we need to connect the outer atoms with the central atoms using single bond or line(-).Every line represent two 【●●】dots or two valence electrons.
Or,
SO2 Lewis Structure Setup Step-3:
Now we have to determine the central atom in SO2.The central atom is that kind of atom that is single or that has lower electronegativity.In case of SO2, S is the central atom and oxygen ,O, is the outer atom as sulfur is less electronegative than than O.
SO2 Lewis Structure Setup Step-4:
Now we need to connect the outer atoms with the central atoms using single bond or line(-).Every line represent two 【●●】dots or two valence electrons.
Or,
In the above structure,we have used 4 valence electrons.So,we have 18-4=14 left.
Now we will use valence electrons to satisfy the outer atoms for completing its octet first.
Or,
In the above structure,we have used 16 valence electrons.So,we have 18-26=2 left.
The remaining 2 electrons will come over central atom now.
Or,
In the above structure,we have used 18 valence electrons.So,we have 18-28=0 left.
Now we need to satisfy the central atom for its octet by using the lone pair from outer atoms.
So, from the above structure we will get two probable structures (A) and( B) in which the sulfur atom follow the octet rule.
The above two structures 【(A),(B)】are the probable SO2 Lewis structures.To find the best SO2 Lewis structure from these ,we have to apply the formula for formal charge.
SO2 Formal Charge
Formal charge =Valence electrons-Dots-Lines
Now we need to calculate the formal charge for each atom of the above two structures.
So,in the above two structures( A and B),the formal charge is not zero but we know that the molecule is neutral.So,we need to try to draw another structure where formal charge will be zero.Remember that sulfur can break octet rule.
Here is the third SO2 Lewis structure (C) where the sulphur stom has more than eight electrons.
Now we need to calculate the formal charge for each atom of the above two structures.
So,in the above two structures( A and B),the formal charge is not zero but we know that the molecule is neutral.So,we need to try to draw another structure where formal charge will be zero.Remember that sulfur can break octet rule.
Rule-4:
Formal Charge =Valence Electrons - unshared Electrons - 1/2 (Shared Electrons)
●The formal charge of sulphur is zero .
•Valence electrons of S =6
•Shared electrons of S= 8
•Unshared electrons of S = 2
●F.C. of S =6- 2 -1/2(8)
=6-2-4
=6-6
=0
●The formal charges of oxygens are also zero.
•Valence electrons of O =6
•Shared electrons of O= 4
•Unshared electrons of O =4
F.C. of O = 6 - 4 -1/2(4)
=6-4-3
=6- 6
=0
 |
| SO2 Lewis structure with formal charges of zero |
So,the best SO2 Lewis dot structure will be the one where most atoms will have zero formal charge.Here is the best Lewis dot structure for SO2(sulphur dioxide).


 |
| Resonance |
Remember that Lewis structure has draw backs.Sometimes,lewis structure can't explain the structure of molecule.To know real structure of SO2,we have to consider resonance hybrid of the above three structures.
◆How many valence electrons does sulfur dioxide SO2 have?
Answer:
Sulfur dioxide, SO2, has 18 valence electrons.
S-->6
2O-->6×2 =12
----------------------
SO2 ------>18
◆The Lewis structure of SO2 (sulfur dioxide) has ____lone pairs of electrons and___ double bonds. (Note that in SO2 the O atoms are not bonded together). What is the correct answer?
Answer:
The Lewis structure of SO2 (sulfur dioxide) has five lone pairs of electrons and two double bonds. (Note that in SO2 the O atoms are not bonded together).
Answer:
Answer:
Is SO2 polar or nonpolar?
Answer:
SO2 is a polar molecule.Again, sulphur dioxide is polar molecule because of its bent shape(V type shape) or geometry which makes the net molecular dipole moment non zero.Again,Since SO2 has net molecular dipole moment,SO2 is polar.
Explanation:
Here are the few steps that you help you to determine that sulphur dioxide,SO2, is a polar molecule.
Step-1:
Draw the Lewis structure of SO2.
Step-2:
After drawing Lewis structure of SO2,now you need to consider the electronegativity difference between atoms.
Here oxygen is more electronegative than sulphur.So,every oxygen-sulphur bond is polar.
Step-3:
Now consider bond dipole moment.
As both oxygen and sulphur bonds are polar,every bond has a dipole moment.
Step-4:
Now consider the geometry or shape of SO2 molecule by applying VSEPR theory.
According to VSEPR theory,we get bent shape or geometry of SO2.
Step-5:
Now consider the net molecular dipole moment.
As the shape of SO2 is bent(V type),one bond dipole moment does not cancel another bond's dipole moment.Thus,we get non zero value for the net molecular dipole moment .As the value of net molecular dipole moment is not zero,we can say that SO2 is polar.















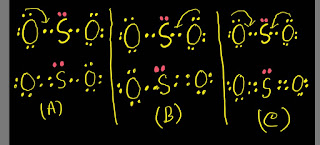












Comments
Post a Comment