【】CO (Carbon Monoxide) Lewis Dot Structure with Formal Charge
CO (Carbon Monoxide) Lewis Structure
Answer:The definition of carbon monoxide (CO) Lewis structure is given below:
CO Lewis structure(carbon monoxide electron dot structure) is that type of structure where we show the total ten valence electrons of CO as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots(●●) but a lone pair of two electrons is shown by dots[●●].
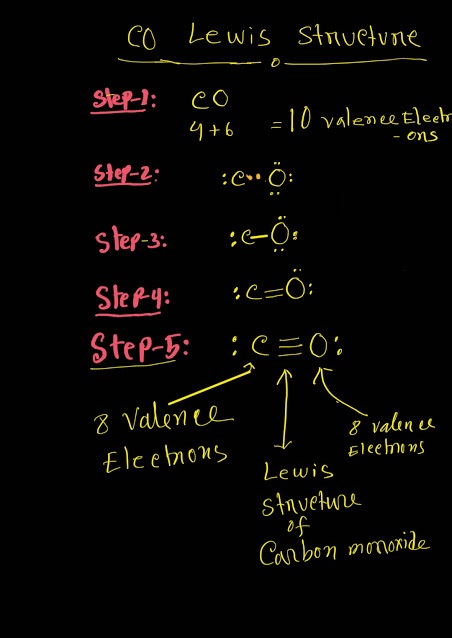
In CO Lewis structure,the carbon atom follows the octet rule and the oxygen atom also follows the octet rule.So,CO follows the octet rule for all the atoms.
In CO Lewis structure,we get five pairs of electrons.Out of five pairs of electrons,CO has three bond pairs and two lone pairs of electrons.Here carbon has one lone pair and oxygen has one lone pair of electrons.
Explanation:
Hello,today I am going to show you the CO Lewis structure in just few easy steps.
CO Lewis Structure Setup Step-01:
To draw the CO Lewis structure, we have to find out the CO valence electrons first.We express valence electrons as dots in lewis dot structure.
To get the valence electrons of carbon,we need to look at the electronic configuration of carbon.
C(6)=1s²2s²2p²
The highest value of principal quantum number here is n=2.
The highest value of principal quantum number ,n , indicates the valence shell and we know the electrons in valence shell is called valence shell.

The nunber of valence electrons in carbon is 4.

Again, we have to find out the valence electrons of oxygen.
To get the valence electrons of oxygen,we need to look at the electronic configuration of oxygen.
O(8)=1s²2s²2p⁴
The highest value of principal quantum number here is n=2.
The highest value of principal quantum number ,n , indicates the valence shell and we know the electrons in valence shell is called valence shell.

The nunber of valence electrons in oxygen is 6.
Another way,carbon is an element of group 4A,so it has four valence electrons.On the other hand, oxygen is an element of group 6A,so it has six valence electrons.
CO Valence Electrons
Now we have to count total number of CO valence electrons .
C = 4
O= 6
So, the total number of valence electrons for CO is ten ( 4+6=10 ).
CO Lewis Structure Setup Step-02:
Now we have to determine the central atom in CO.The central atom is that kind of atom that is single or that has lower electronegativity.In case of CO,Carbon,C, is the central atom and oxygen ,O, is the outer atom as carbon is less electronegetivethan oxygen.
CO Lewis Structure Setup Step-03:
Now we need to connect the outer atoms with the central atoms using single bond or line(-).Every line represent two 【●●】dots or two valence electrons.
In the above structure,we have used 2 valence electrons.So,we have 10-2=8 left.
Now we will use these( left) valence electrons to satisfy the outer atom(O) for completing its octet first.
Or,
In the above structure,we have used 8 valence electrons.So,we have 10-8=2 left.
Now we need to use these 2 electrons to the central atom.
Or,
In the above structure,carbon doesn't have octet.So, to fulfill octet we need to use the lone pair from outer atom oxygen.
So, from the above structure we will see that both atoms have octet.So the Lewis for structure for CO will be the following.
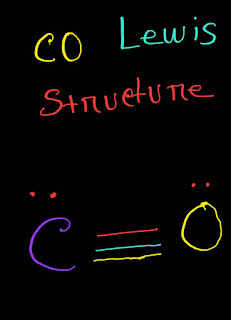
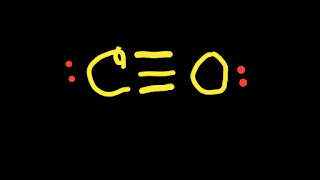
CO Lewis Structure
CO Lewis Structure:Formal Charge
Now we can apply the formula for formal charge for finding formal charge for carbon and oxygen in CO Lewis structure.
Formula -4:
Formal charge=Valence electrons-unbonded electrons-1/2 Bonded electrons
Here ,the formal charge for carbon is 4-2-6/2 =-1
Formula-5:
Formal charge=Valence electrons-unshared electrons-1/2 (shared electrons)
 |
CO (Carbon Monoxide) Lewis Structure |




















CO (Carbon Monoxide) is a colorless gas that is found in the air. It has a complex structure and it can be colorless, odorless or even smelly.
ReplyDeleteIt is produced when burning fuels like coal, oil or natural gas at high temperatures. This process breaks down the molecules of Carbon to CO gas which has no smell or color and forms solid crystals at room temperature. When these crystals are heated up they release more CO gas and thus build up in car catalytic convertors that completely stop the fuel from reaching the engine and thus causing pollution.