【】N2 Lewis Structure ,Valence Electrons,Formal Charge,Polar or Non polar
N2 Lewis Structure
Answer:
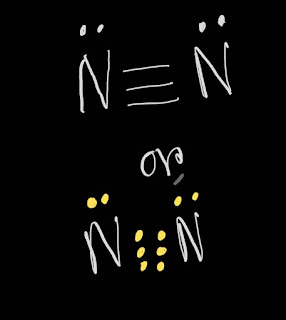
N2 Lewis structure(nitrogen electron dot structure) is that type of structure where we show the total ten valence electrons of N2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) , or dots(●●) but a lone pair of two electrons is shown by dots[●●].
In N2 Lewis structure, there are three bonds between two nitrogen atoms.
●N2 Lewis structure has ten valence electrons.
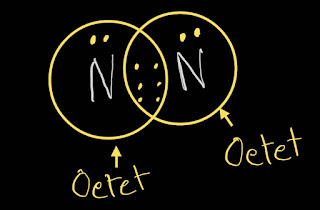
In N2 Lewis structure,both nitrogen follow the octet rule.
In N2 Lewis structure,we get five pairs of electrons.Out of five pairs of electrons, N2 has three bond pairs and two lone pairs of electrons.Here,each nitrogen atom carries one lone pair of electrons.
Explanation:
Hello,today I am going to draw the N2 Lewis structure in just few steps.
Step-1:N2 Valence Electrons
To draw the N2 Lewis structure , we have to find out the valence electrons of nitrogen first.We express valence electrons as dots in lewis dot structure.
To get the valence electrons of nitrogen,we need to look at the electronic configuration of nitrogen.
N(7)=1s²2s²2p³
The highest value of principal quantum number here is n=2.
The highest value of principal quantum number ,n , indicates the valence shell and we know the electrons in valence shell is called valence shell.

The nunber of valence electrons in a nitrogen atom is 5. So N2 has (5×2=10) ten valence electrons totally.
N--> 5
2N-->5×2 =10
Another way, nitrogen is an element of group 5A ,so it has five valence electrons.For N2 ,we get 10 valence electrons.
N2 Lewis structure Setup Step-2:
For a nitrogen molecule ,N₂,the N2 Lewis structure is given below:
Another way, nitrogen is an element of group 5A ,so it has five valence electrons.For N2 ,we get 10 valence electrons.
N2 Lewis structure Setup Step-2:
For a nitrogen molecule ,N₂,the N2 Lewis structure is given below:
N2 has total ten valence electrons.These ten valence electrons have two tasks.First,they connect two nitrogens and then satisfy the octet for them at the same time.
Now put two between them.
So , we have now 10-2= 8 valence electrons.
Now satisfy the octet of a nitrogen atom(Left nitrogen,say).
Now we have 8-6 =2 valence electrons left.
Now put these two valence electrons over the right side nitrogen.
 |
| N2 Lewis Structure |
This is the N2 Lewis structure.In the N2 Lewis structure,we have one triple bond and one lone pair over both nitrogen atom.Both nitrogen atoms satisfy the octet rule.
Continue
N2 Lewis Structure:Formal Charge
Answer:
Formal charge =Valence electrons - Unshared Electrons - 1/2(Shared Electrons)
N ----> 5 - 2 - 1/2(6) =0
N ---->5 - 2 - 1/2 (6) =0
In N2 Lewis structure,the formal charge of every nitrogen atom is zero.
Is N2 Lewis Structure Polar or Non Polar ?
- N2 (Nitrogen molecule) is not polar.There is no polar bond between two nitrogen atoms as there is no electronegativity difference between them.As a result,no bond dipole is produced.So bond dipole moment is zero, and thus the net molecular dipole moment is also zero.So, N2 is not polar .N2 is a non polar molecule.
◆How many bonding electrons are in the Lewis structure of N2?
There are 6 bonding electrons or 3 pairs of bonding electrons in the Lewis structure of N2.
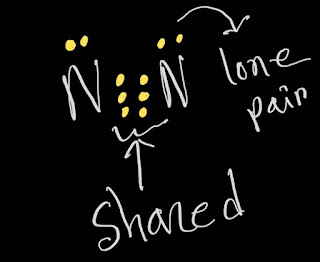















Comments
Post a Comment