【】O2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
O2 Lewis Structure
Answer:O2 Lewis structure (oxygen electron dot structure) is that type of diagram where we show the total 12 valence electrons of O2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots(●●) but a lone pair of two electrons is shown by dots[●●].
In O2 Lewis Dot structure,we get six pairs of electrons.Out of six pairs of electrons,O2 has two bond pairs and four lone pairs of electrons.Here every oxygen atom carries two lone pair of electrons.
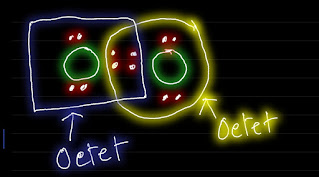
In O2 Lewis structure,the two oxygen atoms follow the octet rule.
Explanation:
Hey!today I am going to show you how to draw the O2 Lewis Dot structure in just two steps.
To draw the O2 Lewis structure , we have to find out the O2 valence electrons first.We express valence electrons as dots in lewis dot structure.
To get the O2 valence electrons ,we need to look at the electronic configuration of oxygen.
O(8)=1s²2s²2p⁴
The highest value of principal quantum number here is n=2.
The highest value of principal quantum number ,n , indicates the valence shell and we know the electrons in valence shell is called valence shell.

The nunber of valence electrons of oxygen atom is 6.And O2 has (6×2 =) 12 (twelve) valence electrons.
Step-1:O2 Valence Electrons
To draw the O2 Lewis structure , we have to find out the O2 valence electrons first.We express valence electrons as dots in lewis dot structure.
To get the O2 valence electrons ,we need to look at the electronic configuration of oxygen.
O(8)=1s²2s²2p⁴
The highest value of principal quantum number here is n=2.
The highest value of principal quantum number ,n , indicates the valence shell and we know the electrons in valence shell is called valence shell.

The nunber of valence electrons of oxygen atom is 6.And O2 has (6×2 =) 12 (twelve) valence electrons.
O2 Lewis Structure Setup Step-2:
Connect two oxygen atom using two valence electrons out of 12 valence electrons.
We have used two valence electrons out of 12.So have 12 -2 = 10 valence electrons left.
Out of 10 valence electrons ,we use 6 valence electrons to satisfy the octet for one oxygen atom(Right here).
Now we have 10 - 6 =4 valence electrons left.Put these four valence electrons over the second oxygen atom.
One oxygen atom has an octet.But the other one doesn't have the octet.
So,we need to make one more bond by moving one lone pair to satisfy the octet rule.
This is the O2 Lewis structure.
O2 Lewis Structure:Formal Charge
Now we need to calculate the formal charge for each oxygen atom of the above O2 Lewis structure.
Here is the formula for determining the formal charge of each atom in O2 Lewis structure.
•Formal charge =Valence electrons - Unshared Electrons - 1/2(Shared Electrons)
•Valence electrons of O (Left) =6
•Shared electrons of O (Left)= 4
•Unshared electrons of O(Left) = 4
●F.C. of O (Left) in O2 =6- 4 -1/2(4)
=6 - 4 - 2
=6 - 6
=0
•Valence electrons of O (Right) =6
•Shared electrons of O (Right)= 4
•Unshared electrons of O(Right) = 4
●F.C. of O (Right) in O2 =6- 4 -1/2(4)
=6 - 4 - 2
=6 - 6
=0
So,the best O2 Lewis structure will be the one where most atoms will have zero formal charge.Here is the best O2 lewis structure .
 |
| Best O2 Lewis structure |
Continue
Some Questions and Answers
●Is O2 polar or nonpolar?
Answer:
















Comments
Post a Comment