【Tips】What is Water (H2O) Made of? ||What is the Formula of Water
What is Water(H2O) Made of?
Many people want to know how water molecule is made out of or made of.Actually, every matter is made of atoms.Water is also made of atoms.
The chemical formula of water is H₂O.So water molecule is made out of hydrogen atom and oxygen atom.In water we get two hydrogen atoms and one oxygen atom.
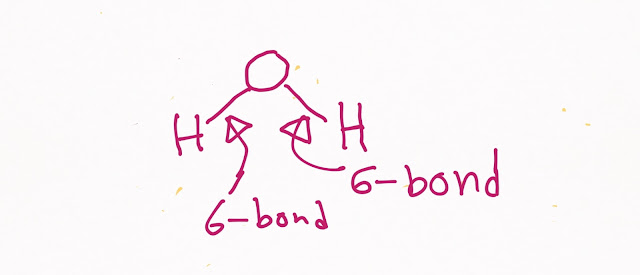 |
| What is water molecule made of |
Water is a polar covalent molecule.In a water molecule ,we get two covalent bonds(H-O-H).
There are billions of water molecules in a single drop of water.Among water,we get hydrogen bond.
 |
| Hydrogen bond |
This bond is responsible for the liquid state of water.
What is the Formula of Water?
-The of water is H₂O
Here 2 is a subscript that indicates that in a water molecule we get two hydrogen atoms.
Next
Characteristics of Hydrogen Bonding







Nice Post.
ReplyDeletehttps://qustionanswer5.blogspot.com/2022/10/blog-post_20.html