【Tips】Hydrogen Bonding in Water||What is H Bonding
Hydrogen Bonding in Water
Hello !everyone ,today I am going to explain the hydrogen bonding in water.You know that water has the atom of Oxygen and hydrogen.The chemical formula of water is H₂O.
The electronegativity of oxygen is higher than hydrogen.
The electronegativity of oxygen is higher than hydrogen.
The electronegativity value of oxygen is 3.5 and the electronegativity value of Hydrogen is 2.3.
The difference between the electronegativity of Oxygen and hydrogen is (3.5 - 2.3 equal to) 1.2.
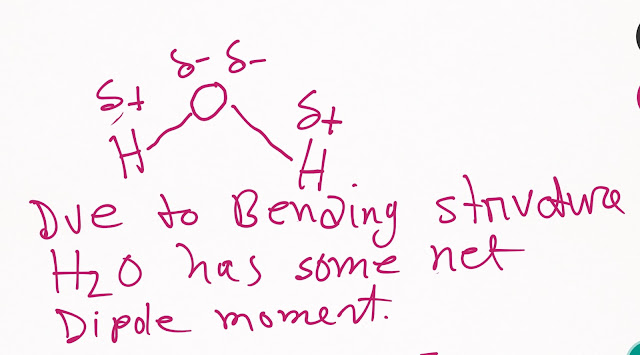
Since the structure of water molecule is bending,the electronegativity difference (1.2) indicates that the bond between hydrogen and oxygen (O-H) in water molecule(H-O-H) is polar.That is, oxygen will be partially negative and hydrogen will be partially positive.
So you can say that H₂O is a polar molecule.
Remember that the electronegativity difference for nonpolar bond is from 0.2 to 0.4 and the electronegativity difference for polar covalent bond is from 0.52 to 1.6 and for ionic bond is more than 1.7.
What is Hydrogen Bond(H-Bond)?
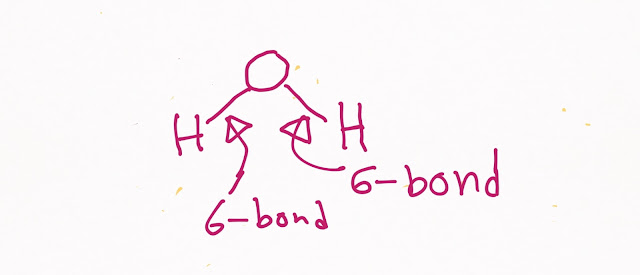
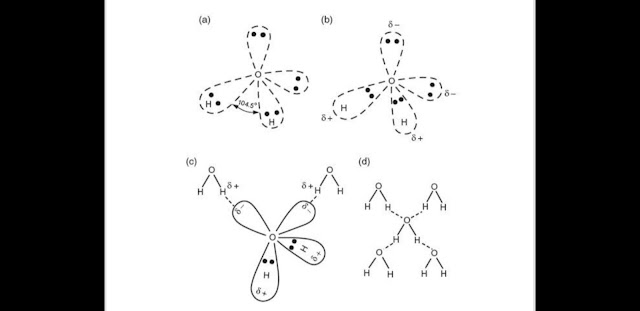
Hydrogen bond is a bridge type of bond in which hydrogen will make bond with two partially electronegative atoms (see picture).Here hydrogen is bonded with two partially negative oxygen atoms.
A single water molecule can form four hydrogen bonds(See picture:two with two hydrogen and two with one Oxygen as oxygen has two lone pairs over it)
Due to the hydrogen bonding in water ,water is liquid and it's boiling point is high. Remember that water in vapour state doesn't contain any hydrogen bond. When we heat water,actually we break the hydrogen bonding in water.









Comments
Post a Comment