【 】CO Lewis Structure ,Valence Electrons ,Formal Charge ,Polar or Nonpolar
CO Lewis Structure
Answer:CO Lewis structure(carbon monoxide electron dot structure) is that type of structure where we show the total ten valence electrons of CO as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots(●●) but a lone pair of two electrons is shown by dots[●●].
 |
| CO Lewis Structure |
In CO Lewis structure,the carbon atom follows the octet rule and the oxygen atom also follows the octet rule.So,CO follows the octet rule for all the atoms.
In CO Lewis structure,we get five pairs of electrons.Out of five pairs of electrons,CO has three bond pairs and two lone pairs of electrons.Here carbon has one lone pair and oxygen has one lone pair of electrons.
Explanation:
Today we are going to learn to draw CO Lewis structure (Carbon monoxide Lewis structure) in just few steps.
CO Lewis Structure Setup Steps
There are 5 steps that you need to follow when drawing the CO Lewis structure.
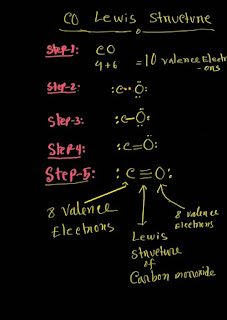
Step-01:CO Valence Electrons
To draw the CO Lewis structure, we have to find out the CO valence electrons first.We express valence electrons as dots in lewis dot structure.
To get the valence electrons of carbon,we need to look at the electronic configuration of carbon.
C(6)=1s²2s²2p²
The highest value of principal quantum number here is n=2.
The highest value of principal quantum number ,n , indicates the valence shell and we know the electrons in valence shell is called valence shell.

The nunber of valence electrons in carbon is 4.

Again, we have to find out the valence electrons of oxygen.
To get the valence electrons of oxygen,we need to look at the electronic configuration of oxygen.
O(8)=1s²2s²2p⁴
The highest value of principal quantum number here is n=2.
The highest value of principal quantum number ,n , indicates the valence shell and we know the electrons in valence shell is called valence shell.

The nunber of valence electrons in oxygen is 6.
Another way,carbon is an element of group 4A,so it has four valence electrons.On the other hand, oxygen is an element of group 6A,so it has six valence electrons.
In CO, Carbon has 4 valence electrons.The oxygen has 6 valence electrons.
 |
| CO Valence Electrons |
Add those all up, you will get CO valence electrons.CO has 10 valence electrons totally.
CO Lewis Structure Setup Step-02
Now we have to identify the central atom .The central atom is that kind of atom that all of the other atoms will be bonded to.The central atom is usually the element that has lower electronegativity or there is only one of.
In case of CO,C is the central atom because it has lower electronegativity than oxygen.Always write the central atom in the middle.
In case of CO,C is the central atom because it has lower electronegativity than oxygen.Always write the central atom in the middle.
CO Lewis Structure Setup Step-03
CO Lewis Structure Setup Steps-04
Put all of the remaining valence electrons on atoms as lone pair.Be careful here, always first fill up the octet of the atom that is not central atom.
For CO,we started with 10 electrons;we have used two electrons for connecting carbon and oxygen .Now we have 8 more electrons left over.
For CO,we started with 10 electrons;we have used two electrons for connecting carbon and oxygen .Now we have 8 more electrons left over.
Out of 8 valence electrons,use six to satisfy the octet of oxygen.
Now you have 8-6 =2 valence electrons left.These two valence electrons will now go to central atom carbon.
See if any atom does not have an octet.
That's right,the Carbon is unhappy because it has only 4 valence electrons.
So,what can we do to make it happy?We can't just give it more electrons,since we don't have any more.Instead,the oxygen atom can take two pairs(4 electrons)out of its(C) three lone pairs and share those 4 electrons with Carbon in another two bonds.
So,what can we do to make it happy?We can't just give it more electrons,since we don't have any more.Instead,the oxygen atom can take two pairs(4 electrons)out of its(C) three lone pairs and share those 4 electrons with Carbon in another two bonds.
Now the triple bond or six valence electrons between Oxygen and Carbon makes every atom happy with 8 valence electrons.So this is the Lewis structure of CO.
 |
| The lewis structure of CO including lone pairs and formal charges. |
 |
| Lewis Structure of Carbon monoxide
Look at the picture.
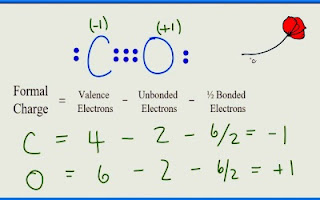
CO Lewis Structure:Formal ChargeNow we can apply the formula for formal charge for finding formal charge on every atom of CO Lewis Structure.
Formula-1: Formal Charge=Valence Electrons-Dots-Lines Formula-2: Formula-3: Formal charge=Valence electrons-unshared electrons-1/2 (shared electrons) The formal charge for oxygen is 6-2-6/2= +1 |
 |
| How do you draw the Lewis structure for CO? |
Some Questions and Answers Related to CO Lewis Structure
Draw the Lewis structure of CO and include lone pairs and formal charges.
Answer:
This is the Lewis structure of CO including lone pairs and formal charges.
●What is the formal charge of carbon in the best Lewis structure of CO?
Answer:
The formal charge of carbon in the best Lewis structure of CO is -1.
●What is the formal charge of oxygen in the best Lewis structure of CO?
Answer:
The formal charge of oxygen in the best Lewis structure of CO is +1.
●Does carbon monoxide have a double bond in its Lewis structure?
Answer:
No,carbon monoxide,CO, doesn't have a double bond in its Lewis structure.
◆ Is CO polar or nonpolar?
Answer:
In the case of CO, the oxygen is more electronegative than carbon.That is why carbon-oxygen bond is polar.So this has bond dipole moment.
According to VSEPR theory,the shape of CO is linear.So,we are not getting anything that can cancel the bond dipole moment of carbon-oxygen bond of linear CO molecule. Thus the net molecular dipole moment for CO molecule is not zero.Therefore ,CO (Carbon monoxide) is polar.







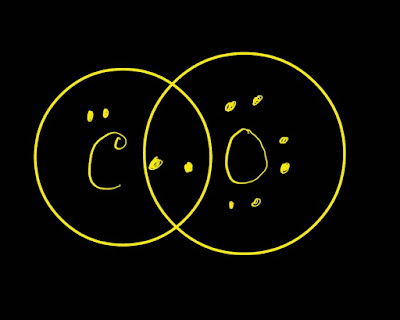












The CO (Carbon Monoxide) Lewis Dot Diagram is a simple and effective tool for identifying dangerous levels of carbon monoxide in the home. It is commonly used to avoid poisoning.
ReplyDelete