In the ground-state electron configuration of Fe3+, how many unpaired electrons are present?
In the ground-state electron configuration of Fe3+, five unpaired electrons are present.
Explanation:
To find out the number of unpaired electrons in Fe^3+ ion, you need to write the electron configuration of Fe3+.Remember that the atomic number of iron is 26.
Here is the ground state electron configuration of Fe^3+:
[Ar]3d⁵
Remember that the d subshell has five orbitals.Each orbit can take maximum two electrons.
In case of filling degenerate orbits ,each orbital will take one electron before pairing by the second electron according to Hunds' rule.
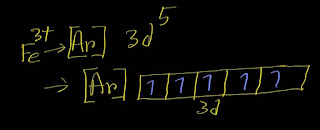







Comments
Post a Comment