How many unpaired electrons are present in Fe, Fe2+ and Fe3+?
In the ground-state electron configuration of iron, Fe, four unpaired electrons are present.
Explanation:
To find out the number
of unpaired electrons in Fe, you need to write the electron configuration of Fe.Remember that the atomic number of iron is 26.
Here is the ground state electron configuration of Fe:
[Ar]3d⁶4s²
Remember that the d subshell has five orbitals.Each orbit can take maximum two electrons.
In case of filling degenerate orbits ,each orbital will take one electron before pairing by the second electron according to Hunds' rule.
In the ground-state electron configuration of Fe2+, four unpaired electrons are present.
Explanation:
To find out the number of unpaired electrons in Fe^2+ ion, you need to write the electron configuration of Fe^2+.
Remember that the atomic number of iron is 26.
Here is the electron configuration of Fe^2+:
[Ar]3d⁶
Remember that the d subshell has five orbitals.Each orbit can take maximum two electrons.
In case of filling degenerate orbits ,each orbital will take one electron before pairing by the second electron according to Hunds' rule.
In the ground-state electron configuration of Fe3+, five unpaired electrons are present.
Explanation:
To find out the number of unpaired electrons in Fe^3+ ion, you need to write the electron configuration of Fe3+.Remember that the atomic number of iron is 26.
Here is the electron configuration of Fe^3+:
[Ar]3d⁵
Remember that the d subshell has five orbitals.Each orbit can take maximum two electrons.
In case of filling degenerate orbits ,each orbital will take one electron before pairing by the second electron according to Hunds' rule.

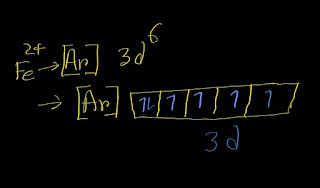
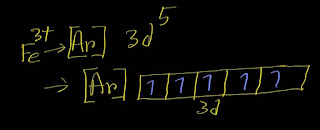







Comments
Post a Comment