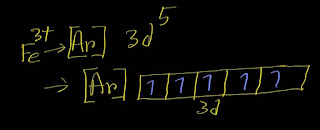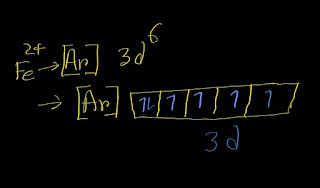What is the name of the ion Fe3+?
Iron(III) ion Explanation: The systematic name(IUPAC) of Fe^3+ cation is iron(III) ion. Step-1: First,write the name of the element Fe. Fe is called iron. Step-2: Now put the oxidation number of Fe within parentheses, ( ). Iron(III) Now you have got the systematic nomenclature of Fe^3+. Now I going to give the old name of Fe^3+. The Latin name for iron element is ferrum.The charged iron ion with the highest oxidation number (3+) is named as ferric ion and the lower charged iron ion (2+) was is named as ferrous ion.