【 】Is co2 polar or nonpolar ?
Is CO2 polar or nonpolar?
Answer:
CO2 is a nonpolar molecule.Again,Carbon dioxide is nonpolar because of its linear geometry which makes the net molecular dipole moment zero.
Explanation:
Here are the few steps that you help you to determine that carbon dioxide,CO2, is a non polar molecule.
Step-1:
Draw the Lewis structure of CO2.
Step-2:
After drawing Lewis structure of CO2,now you need to consider the electronegativity difference between atoms.
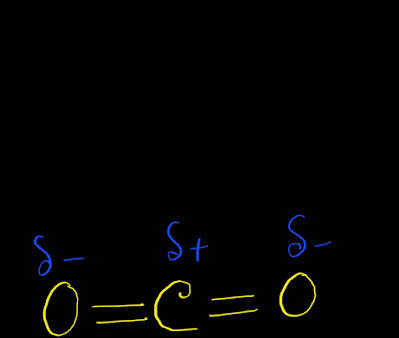
Here oxygen is more electronegative than carbon.So,every carbon-oxygen bond is polar.
Step-3:
Now consider bond dipole moment.
As both carbon and oxygen bonds are polar,every bond has a dipole moment.
Step-4:
Now consider the geometry of CO2 molecule by applying VSEPR theory.
According to VSEPR theory,we get linear geometry of CO2.
Step-5:
Now consider the net molecular dipole moment.
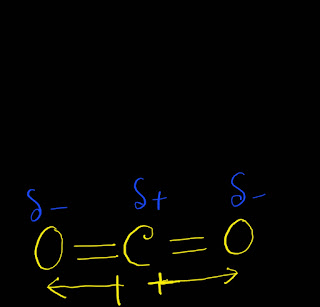
As the shape of CO2 is linear,one bond dipole moment cancel another bond's dipole moment.Thus,we get zero value for the net molecular dipole moment .As the value of net molecular dipole moment is zero,we can say that CO2 is nonpolar.








Comments
Post a Comment