【 】 1s2 2s2 2p6 ||What is 1s2 2s2 2p6?||What element has the electron configuration of 1s2 2s2 2p6?
What is 1s2 2s2 2p6 ?
Answer:
1s2 2s2 2p6 means that
◆s subshell of first orbit or energy level has two electrons,
◆s subshell of second orbit or energy level has two electrons,
●and p subshell of second orbit or energy level has six electrons.
Read:CO2 Lewis Structure
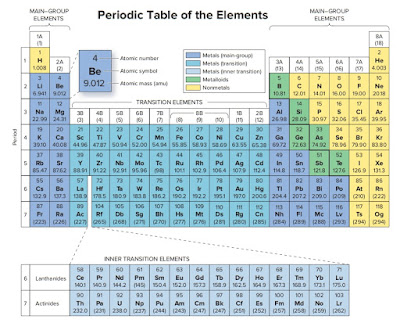
Now if you look at the periodic table,Ne has atomic number 10.So
1s2 2s2 2p6 is electron configuration of Neon(Ne).
Read:CO2 Lewis Structure
From 1s2 2s2 2p6 electron configuration,we get 2+2+6 = 10 electrons.
We know that electron's number is equal to proton number or atomic number for neutral atom.Since here there is no words about charge,we can say that 1s2 2s2 2p6 indicates the element whose atomic number is 10.
Now if you look at the periodic table,Ne has atomic number 10.So
1s2 2s2 2p6 is electron configuration of Neon(Ne).

•What element is best represented by the electron configuration 1s2 2s2 2p6?
Answer: The element Neon,Ne,is the best represented by the electron configuration 1s2 2s2 2p6.
•How many electrons does 1s2 2s2 2p6 have?
Answer:
1s2 2s2 2p6 has ten electrons.
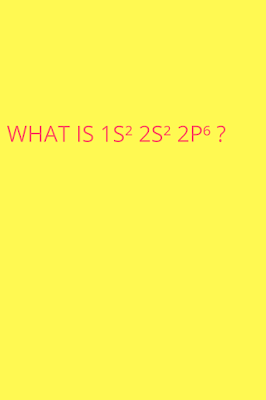







Comments
Post a Comment