【 】Is NH3 Polar or Nonpolar?||Is Ammonia a Polar or Nonpolar Molecule?
Answer:
NH3(ammonia) is a polar molecule.For asymmetrical shape of NH3,the net dipole moment or molecular dipole moment of NH3 molecule is not zero.This indicates that NH3 is polar.
Explanation:
Hello!today I am going to tell you whether NH3 is polar or nonpolar molecule in just few steps.
Step-1:
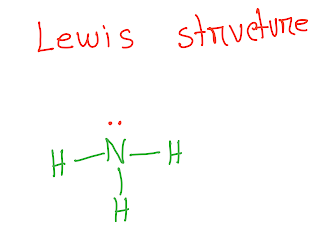
Write the Lewis structure of NH3 first.You know that in ammonia there are 8 valence electrons.Five has come from nitrogen as it the member of group 5A and 3 valence electrons from 3 H atoms.Each hydrogen atom provide one electron as H is the member of group 1A.
From the lewis structure of NH3 ,it is clear that nitrogen atom has one lone pair electron.
Now consider electronegativity difference.In NH3,every N-H bond is polar because nitrogen is more electronegative than hydrogen.
 |
Is NH3 Polar or Nonpolar? |
Step-1:
Write the Lewis structure of NH3 first.You know that in ammonia there are 8 valence electrons.Five has come from nitrogen as it the member of group 5A and 3 valence electrons from 3 H atoms.Each hydrogen atom provide one electron as H is the member of group 1A.
From the lewis structure of NH3 ,it is clear that nitrogen atom has one lone pair electron.
Now consider electronegativity difference.In NH3,every N-H bond is polar because nitrogen is more electronegative than hydrogen.
Now consider the bond dipole moment.As all N-H bonds are polar due to the electronegativity difference,all three N-H bonds have bond dipole moment.
Now you need to know whether these bond dipole moments are canceling each other or not from the geometry of the molecule.
Step-2:
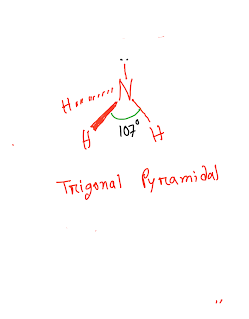
Now you have to apply VSEPR theory to predict the shape or geometry of NH3.
According to this theory(3 bond pairs +1 lone pair around central atom N),the shape of NH3 is trigonal pyramidal[Bond angeles with central atom is 107°approx.].This shape is asymmetrical.Asymmetrical means both sides of the molecule are not identical.
Step-3:
Now you need to consider the net molecular dipole moment.
Step-2:
Now you have to apply VSEPR theory to predict the shape or geometry of NH3.
According to this theory(3 bond pairs +1 lone pair around central atom N),the shape of NH3 is trigonal pyramidal[Bond angeles with central atom is 107°approx.].This shape is asymmetrical.Asymmetrical means both sides of the molecule are not identical.
Step-3:
Now you need to consider the net molecular dipole moment.
For asymmetrical shape of NH3,the net dipole moment or molecular dipole moment of NH3 molecule is not zero.This indicates that NH3 is polar as 3 bonds' dipole moment (N-H) don't cancel each other.
Step-4:
Notice that the dipole moments of bond dipoles(3 N-H) are vectors .So you need to consider the direction of dipole moments.The direction is from lower electronegative atom to more electronegative atom.
The overall polarity of a molecule depends on the vectoral sum of the dipole moments of bond dipoles .
The bond dipole moments vectors may add together to give net molecular dipole moments , or they may cancel out , resulting in a polar molecule or a nonpolar molecule .
That'sOK. Just because a molecule has polar bonds does not make sure that the molecule is polar .You have to look at the net dipole moment or molecular dipole moment.
Remember that the molecular dipole moment or net dipole moment is the resultant of all of the individual bond dipole(3 N-H) moments of a substance(NH3).
Notice that molecules that consist of three or more atoms are generally polar unless the following condi tion is met.
lf the contral atom has no lone pairs and is surrounded by atoms of one element ,then the molecule will be non polar(CO2,CH4,CCl4).As NH3 has has three atoms and central atom(N) has one lone pair,so it will be polar.
Step-4:
Notice that the dipole moments of bond dipoles(3 N-H) are vectors .So you need to consider the direction of dipole moments.The direction is from lower electronegative atom to more electronegative atom.
The overall polarity of a molecule depends on the vectoral sum of the dipole moments of bond dipoles .
The bond dipole moments vectors may add together to give net molecular dipole moments , or they may cancel out , resulting in a polar molecule or a nonpolar molecule .
That'sOK. Just because a molecule has polar bonds does not make sure that the molecule is polar .You have to look at the net dipole moment or molecular dipole moment.
Remember that the molecular dipole moment or net dipole moment is the resultant of all of the individual bond dipole(3 N-H) moments of a substance(NH3).
Notice that molecules that consist of three or more atoms are generally polar unless the following condi tion is met.
lf the contral atom has no lone pairs and is surrounded by atoms of one element ,then the molecule will be non polar(CO2,CH4,CCl4).As NH3 has has three atoms and central atom(N) has one lone pair,so it will be polar.
Read More:







Comments
Post a Comment