【】Oxidation of Ethene or Ethylene (CH2=CH2)
Oxidation of Ethene or Ethylene(CH2=CH2)
Solution:
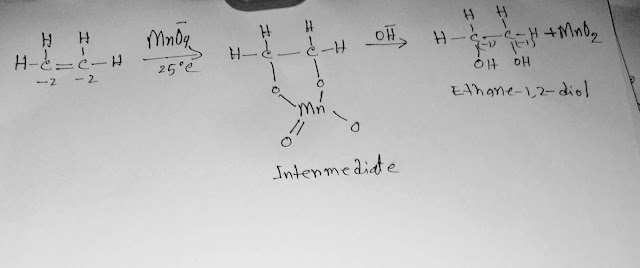
Today we will talk about the oxidation of ethene.
In ethene, the oxidation number of every carbon is -2.

When we apply mild oxidising conditions to ethene,we get ethane-1,2-diol.
In mild oxidising conditions, the oxidising agent is kept cold at room temperature.
For mild oxidation of ethene,we use alkaline solution of KMnO4 (potassium permanganate).
In the above reaction, ethene has lost electrons,so ethene has oxidized.Look at the change of oxidation number from -2 to +4.









how is diol form
ReplyDelete