【】How Many Neutrons Does Hydrogen Have?||Number of Neutrons in Hydrogen
How Many Neutrons Does Hydrogen Have?
Answer:
Hydrogen has zero neutrons for this isotope.
Explanation:
Hello,Everyone! today we are going to learn about how many neutrons hydrogen have in just three steps.
To find out the number of neutrons for hydrogen,we have to have knowledge about atomic number,atomic mass,mass number of hydrogen.
Step-1:
●The atomic number of hydrogen is the number of protons in hydrogen's nucleus.
●The atomic mass of hydrogen is the average number of protons plus neutrons of all the isotopes of hydrogen.
●The Masss number of hydrogen is the number of protons plus neutrons of a specific isotope of hydrogen.
Step-1:
●The atomic number of hydrogen is the number of protons in hydrogen's nucleus.
●The atomic mass of hydrogen is the average number of protons plus neutrons of all the isotopes of hydrogen.
●The Masss number of hydrogen is the number of protons plus neutrons of a specific isotope of hydrogen.
Step-2:
We need to convert atomic mass of hydrogen to mass number of hydrogen by rounding .
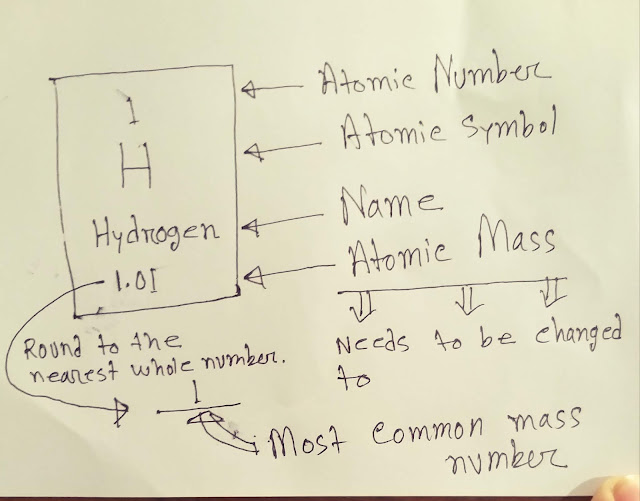
From periodic table,
●Atomic number of hydrogen is 1(one).
●Atomic mass of hydrogen is 1.01
●To find out mass number,I need to round 1.01.So,by rounding,we are getting mass number of hydrogen.The mass number of hydrogen is 1.
Step-3:
By the difference between mass number and atomic number of hydrogen,we get the number of neutrons in hydrogen.
●Number of neutrons=1-1=0
Therefore,the number of neutrons in hydrogen is zero here for this isotope.
● Number of electrons in hydrogen is 1
●Number of protons in hydrogen is 1
Actually,hydrogen has no neutron, But the isotope of hydrogen called deuterium that has one neutron, and called tritium that has two neutrons. Their symbols are therefore ¹₁H, ²₁H, and ³₁H.
Continue









Comments
Post a Comment